Obstructive sleep apnea syndrome is a prevalent sleep disorder involving various domains of quality of life that require an objective evaluation. The aim of the present study was to validate a specific measure of health related quality of life in Portuguese sleep apnea patients—the SAQLI.
Materials and methodsA sample of 206 Portuguese sleep apnea patients completed a Portuguese version of SAQLI for assessment of its comprehensibility, construct validity and reliability and repeated the same measures after 1–3 months CPAP therapy. Hospital Anxiety and Depression Scale (HADS) was also used to test construct validity.
ResultsCronbach alpha coefficients of internal validity were above the standard (0.70) in all domains. Factor analysis showed that the items of daily functioning, emotional and symptoms domains all loaded on the hypothesized scales. Longitudinal data showed a significant difference (p<0.001) in each SAQLI domain and between total score before and after CPAP use. In terms of external validity, all SAQLI domains were negatively correlated with anxiety and depression scales.
ConclusionsOverall the results of the present study demonstrated that the Portuguese version of SAQLI to be psychometrically valid and applicable for evaluate the impact of sleep apnea and CPAP treatment in Portuguese patients.
A Síndrome da Apneia Obstrutiva do Sono é a patologia do sono mais prevalente e afeta os vários domínios de qualidade de vida destes doentes, sendo necessária a sua avaliação objetiva. O objetivo do presente estudo foi o de validar, para a população portuguesa, uma medida específica de qualidade de vida relacionada com a apneia do sono - o SAQLI.
Material e métodosUma amostra de 206 pacientes portugueses com apneia do sono completou a versão portuguesa do SAQLI para avaliação da sua compreensibilidade, validade de construto e confiabilidade, e repetiu as mesmas medidas após um e três meses de terapia com CPAP. A medida de ansiedade e depressão hospitalares (HADS) foi também utilizada para testar a validade de construto.
ResultadosOs resultados de validade interna revelados pelo coeficiente de alfa de Cronbach foram acima do padrão (0,70) para todos os domínios. A análise fatorial mostrou que os itens de funcionamento diário, emocional e sintomas saturaram nos domínios originais da escala. Os dados longitudinais revelaram uma diferença significativa (p < 0,001) em cada domínio do SAQLI e entre o resultado total, antes e após o uso do CPAP. Em termos de validade externa, todos os domínios SAQLI se correlacionaram negativamente com as escalas de ansiedade e depressão.
ConclusõesOs resultados do presente estudo demonstraram que a versão em português do SAQLI apresenta uma boa qualidade psicométrica e é válida para avaliar o impacto da apneia do sono e tratamento com CPAP em pacientes portugueses.
Obstructive sleep apnea syndrome (OSAS) is a very widely spread sleep-related breathing disorder in adults. The estimated prevalence, in Western countries, ranges from 2 to 4% in men to 1 to 2% in women.1,2 OSAS is a respiratory problem causing recurrent episodes of partial and/or complete upper airway obstruction, and results in apnea hypopnea index (AHI) that occur more than five times per hour.3 Patients usually develop two cardinal symptoms: excessive daytime sleepiness and loud snoring witnessed by the bed partner. Other symptoms are also important to support the diagnosis of OSAS, such as nocturnal choking or gasping, nocturia, impotence, fatigue, morning headaches, memory impairment.4 A clear relationship between OSAS and hypertension has been consistently demonstrated,5 and it is also a risk factor for the development of cardiovascular diseases.6 OSAS has been also associated with a decrease of work productivity,7 social problems,8 reduced participation and enjoyment in everyday activities9 and marital problems.10 Important consequences of sleepiness are car and work accidents.11
Reduction in quality of life has been found associated to depression in patients with severe OSAS.12 In a study conducted by Ye et al.,13 anxiety was found to be the strongest predictor of quality of life (measured with SAQLI), when compared with depression, reflecting the effect of mood disturbances on daily activities. Daytime sleepiness is also an incapacitating symptom of OSAS that can lead to substantial impairment in quality of life.13,14
The increasing need for diagnostic services and treatment of OSAS requires valid measures to assess the extent of sleep apnea and effectiveness of treatment. Given the prevalence of OSAS and its negative consequences on physical, social and psychological functions, health related quality of life measures are crucial and would constitute a clinically significant outcome. The majority of studies used multiple instruments to measure quality of life in OSAS patients which may not be able to detect all the important effects of the disease.15 Studies have been published that have measured quality of life with generic instruments, such as SF-36, NHP and SIP.16 SF-36, the most commonly used, has shown that quality of life is markedly impaired with OSAS.17–19 This holds true when using the SAQLI which specifically assesses quality of life in sleep apnea patients.20 In a systematic review, by Moyer et al.,21 impairment of quality of life is well documented in OSAS patients and the authors suggest SAQLI as a potentially useful instrument. Other OSAS health related quality of life instruments which have been used are, Functional Outcomes of Sleep Questionnaires (FOSQ),22 Obstructive Sleep Apnea Patient-Oriented Severity Index (OSAPOSI)23 and Quebec Sleep Questionnaire (QSQ).24 A study that compared content covered by the above mentioned instruments using the international Classification of Functioning, Disability and Health, concluded that if what is important is the success of treatments and side effects then SAQLI would be a good choice.25
In Portugal there are a few studies measuring HQOL (Health related Quality of life) in OSAS and these studies chose to use SF-36 to measure quality of life.17,26 The need to use specific measures of quality of life such as SAQLI in settings with OSAS patients seems important.
SAQLI was developed by Flemons and Reimer, in 1998, to record the fundamental components of OSAS disorder.15 In the process of developing the instrument, the authors consulted sleep apnea experts, carried out structural interviews with OSAS patients and their partners and achieved a fundamental literature review. Flemons and Reimer15 suggest that SAQLI is the only OSAS quality of life instrument that includes the potential negative consequences of the treatment, i.e. emphasizes the evaluation of CPAP and laser assisted uvolopalatoplasty. SAQLI has been shown to be valid to evaluate sleep apnea OSAS patients in western countries.27,28 The issue of cross-cultural adaptability seems to be no longer a problem and as Lacasse et al.27 emphasized, a careful translation and back translation of quality of life questionnaires can produce non-English language versions that appear to operate in a very similar manner to their originals.
The aim of the present study was to evaluate the psychometric properties of SAQLI in a sample of Portuguese OSAS patients.
MethodsSampleDuring an 18 month period, 206 consecutive patients, referred to the Sleep Disordered Breathing Clinic of a University Hospital in Northern Portugal, who had been diagnosed with OSAS and recommended for CPAP treatment, followed a standardized protocol that included a general clinic evaluation by a sleep specialist, a home sleep study and a psychological evaluation that included an assessment of quality of life (SAQLI), anxiety and depression (HADS) and subjective somnolence (Epworth scale) (Table 1). Patients repeated the same tests after 1–3 months of CPAP therapy. None of the participants had a psychiatric disorder or any other sleep disorder. The study was approved by the hospital research ethics committee and written informed consent was obtained from all participants before participation in the study.
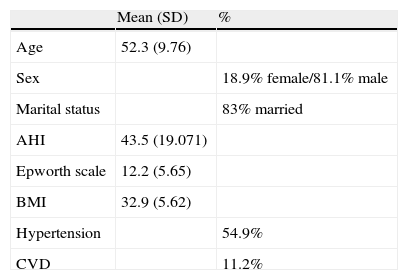
Socio-demographic and clinical characterization of patients (n=206).
| Mean (SD) | % | |
| Age | 52.3 (9.76) | |
| Sex | 18.9% female/81.1% male | |
| Marital status | 83% married | |
| AHI | 43.5 (19.071) | |
| Epworth scale | 12.2 (5.65) | |
| BMI | 32.9 (5.62) | |
| Hypertension | 54.9% | |
| CVD | 11.2% |
AHI – apnea hypopnea index; BMI – body mass index; CVD – cardiovascular diseases.
The sample was composed of 167 males and 39 female OSAS patients, between 27 and 75 years old, with a mean age of 52 years (SD=10). Nineteen of the patients dropped out at the time of the second assessment. Out of 187, 131 patients adhered to CPAP treatment and 54 patients were non-adherent (two patients did not have registered compliance due to download problems with their CPAP memory cards and therefore were not followed in the second phase).
43% of the patients had four years of education and were employed (61%). They presented an average BMI of 33. Almost 55% of patients had hypertension and 11% had cardiovascular diseases. 71% of all OSAS patients had an AHI higher than 30 apneas plus hypopneas per hour of estimated sleep time, after an overnight sleep study using a five-channel recording device (Sleep Screen, Viasys ™ Healthcare). This device produces a computerized recording of variations in oronasal airflow (measured by nasal cannula), body position, wrist oximetry, pulse rate and arterial oxygen saturation (measured by finger pulse oximetry). Sleep technicians carried out a manual analysis of the recordings, by counting apnea (episodes of ≤10% of previous airflow with at least 10s of duration) and hypopnea episodes (episodes showing 20–50% of the previous airflow, with at least 10s of duration together with a 4% dip in oxygen saturation), dividing the total number of these episodes by the sleep time in hours, obtaining a manual respiratory disturbance index according to established criteria.3 Patients were prescribed CPAP by an experienced sleep physician and instructed in the proper use by an experienced therapist, to whom patients had access for 7 days per week to resolve any problems that might occur during use.
InstrumentSAQLI evaluates four domains of quality of life critical to this population and an additional domain to evaluate the effect of treatment: daily functioning (11 items); social interactions (13 items); emotional functioning (11 items); symptoms (5 items); treatment related symptoms (5 items, used in the second evaluation moment). The low end of the scale reveals the greatest impairment1,2 and the high end of the scale reveals the least impairment.6,7
The SAQLI has five domains and questions are equally weighted. In terms of scoring, each question ranges from 1 (greatest impairment) to 7 (least impairment). For each of the first four domains – A–D [A: daily functioning (11 items); B: social interactions (13 items); C: emotional functioning (11 items); D: symptoms (5 items)] – the mean score was obtained by dividing the sum of all the items, within the domain, by the number. The total score is calculated by summing the four domains (A–D) and then dividing by four. Thus, each of the domain scores and the total SAQLI has a range of 1–7, making the scores easier to interpret.15 After treatment, domain E is used and its mean score should be subtracted from the other domains totals, after being recoded – as 0 if the initial score is 7 and as 6 if the initial score is 1. For the symptoms domain and treatment related symptoms domain (domain E), patients may select up to 5 symptoms and the mean score is obtained by summing the scores for the five symptoms and dividing by five (even when the patient selects fewer than five symptoms). Section F measures the impact of treatment related symptoms (domain E) and the benefits of treatment (as reflected by any improvement in quality of life – domain A, B, C and D), drawing a vertical line through a 10cm visual analog scale. Domain E is divided by the impact score of the improvement in quality of life of domain A, B, C and D to provide a “weighting factor” result (with a maximum of 1.0) that is multiplied by the mean score for treatment related symptoms.15 The product is then subtracted from the total of mean scores from the first four domains giving the minimum important difference with a cut off point of 0.5 when a 7 item likert scale is used.15 Higher scores indicated better quality of life.
SAQLI presents a high internal consistency as reflected by the Cronbach's alpha coefficients of 0.88–0.92.15 To determine the measurement properties of SAQLI the authors presented, in 2002, new data.20 Repeatability of the SAQLI at one and two weeks showed a reliability (intraclass correlation) coefficient of 0.92 and did not change between the two weeks. Moderate to strong correlations were observed between SAQLI and other quality of life measures.
ProcedureFor the development of the Portuguese version of SAQLI, the translation and cultural adaptation group (TCA Group)’s guidelines for translation and adaptation of self-report instruments were followed.29 After receiving, from the authors, the authorization and the original articles with the development and measurement properties of SAQLI, the original English version was first translated into Portuguese by two independent native Portuguese speakers. The back translation was performed by an outsourced translator, who was not linked to the research group and did not know the original instrument and its first translations. The discrepancies between the original English version, the translated version into Portuguese and the back translation version were analyzed and only minor differences were found. The final Portuguese version of SAQLI was applied to five OSAS patients, native in Portuguese language to test for comprehension and suggestions. The main suggestion had to do with the format of the instrument, namely to change the assembled color coded cards to colored symbols in the patient's format sheet, which was done.
OSAS patients were invited to participate into the study after the overnight sleep study and the prescription of CPAP by their sleep physician. The information on demographic and clinical variables was obtained by self report and a case record clinic form. SAQLI was administrated by a psychologist. Prior to the start of the interview, the psychologist ensured that the patient fully understood the seven-point likert scales, i.e. the numbers 1–7 represented all possible answers. First SAQLI was used to measure the patient's baseline QOL.15 After that, the psychologist emphasized that the low end of the scale7 represented the least impairment and the high end of the scale1 represented the greatest impairment, while explaining the symbol in the patient's format SAQLI sheet. For all participants, the psychologist read the instructions and the questions while the patient filled in the responses on the questionnaire sheet.
The second moment of the study occurred 1–3 months after CPAP therapy. SAQLI, with all domains plus Domain E (treatment related symptoms) was administered.15 The electronic records of CPAP compliance were analyzed after downloading the therapy details from the CPAP software. Adherence was defined as a CPAP use ≥4h/night, following the cut-off point state in the majority of studies and base on a frequency over >70% of the total treatment days.30,31 After intervention, SAQLI was given to the same OSAS patients.
Data analysisInternal consistency was assessed by Cronbach's alpha. Content validity was analyzed through Pearson correlations of each item with the total of the respective subscale and with the total score, before and after CPAP therapy. A varimax rotation was used to perform the factor analysis.
The evaluative properties of SAQLI were examined, namely the extent the instrument captures changes in quality of life over time.32 This was tested using paired t-tests to detect statistically significant differences in scores in OSA patients that adhere to CPAP treatment over the study period. Pearson correlation coefficients between SAQLI interdomain before and after CPAP treatment and clinical variables were also performed. Construct validity with interdomain correlations between SAQLI and HADS (Hospital Anxiety and Depression Scale) were also performed.
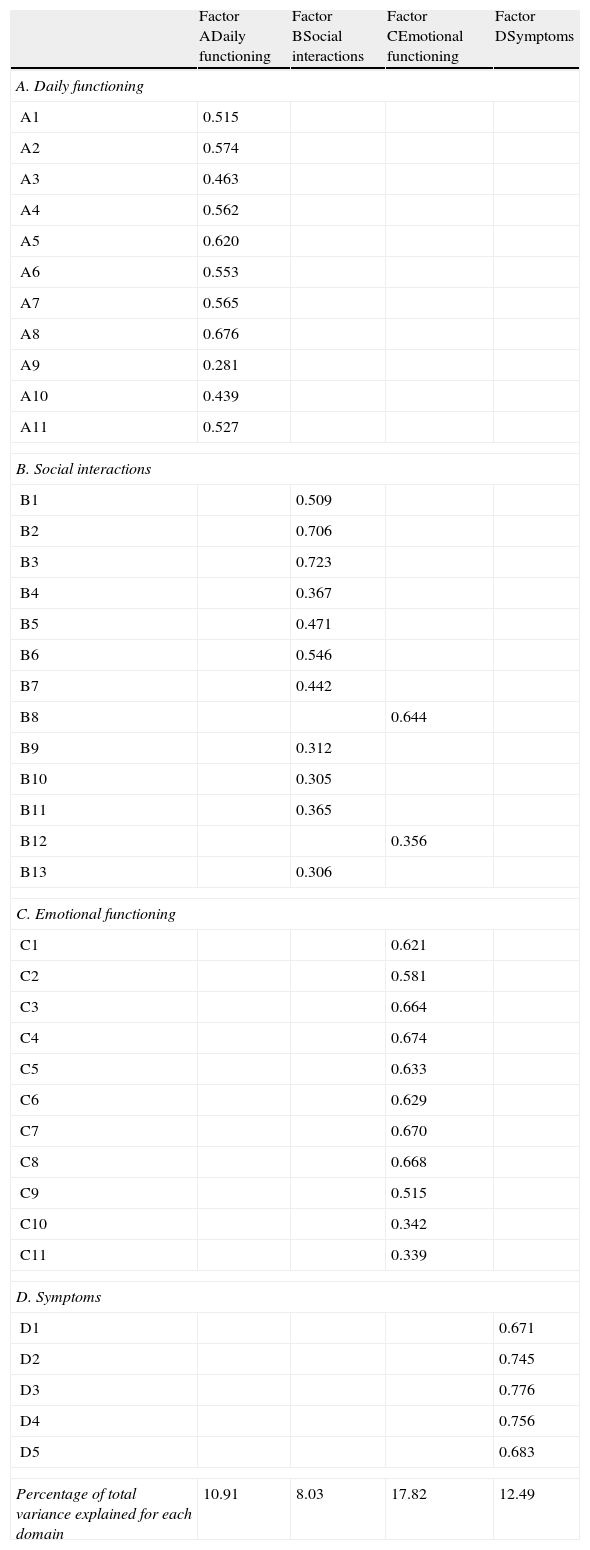
ResultsConstruct validity and fidelity of the Portuguese versionConstruct validity was assessed by performing factor analysis with varimax rotation method.33 The suitability of factor analysis by checking the existence of significant correlations, between the items, was confirmed by Kaiser–Meyer–Olkin (KMO=0.896) and by the Sphericity Bartlett test (QQ=4533; gl=780; p<0.001). Four factors with eigenvalues greater than 1.0 were extracted accounting for 49, 25% of total variance. Factor loadings are shown in Table 2.
Factor loading on SAQLI (n=206).
| Factor ADaily functioning | Factor BSocial interactions | Factor CEmotional functioning | Factor DSymptoms | |
| A. Daily functioning | ||||
| A1 | 0.515 | |||
| A2 | 0.574 | |||
| A3 | 0.463 | |||
| A4 | 0.562 | |||
| A5 | 0.620 | |||
| A6 | 0.553 | |||
| A7 | 0.565 | |||
| A8 | 0.676 | |||
| A9 | 0.281 | |||
| A10 | 0.439 | |||
| A11 | 0.527 | |||
| B. Social interactions | ||||
| B1 | 0.509 | |||
| B2 | 0.706 | |||
| B3 | 0.723 | |||
| B4 | 0.367 | |||
| B5 | 0.471 | |||
| B6 | 0.546 | |||
| B7 | 0.442 | |||
| B8 | 0.644 | |||
| B9 | 0.312 | |||
| B10 | 0.305 | |||
| B11 | 0.365 | |||
| B12 | 0.356 | |||
| B13 | 0.306 | |||
| C. Emotional functioning | ||||
| C1 | 0.621 | |||
| C2 | 0.581 | |||
| C3 | 0.664 | |||
| C4 | 0.674 | |||
| C5 | 0.633 | |||
| C6 | 0.629 | |||
| C7 | 0.670 | |||
| C8 | 0.668 | |||
| C9 | 0.515 | |||
| C10 | 0.342 | |||
| C11 | 0.339 | |||
| D. Symptoms | ||||
| D1 | 0.671 | |||
| D2 | 0.745 | |||
| D3 | 0.776 | |||
| D4 | 0.756 | |||
| D5 | 0.683 | |||
| Percentage of total variance explained for each domain | 10.91 | 8.03 | 17.82 | 12.49 |
Percentage of total variance explained for the 4 extracted domains=49.29%; KMO=0.896; Sphericity Bartlett test: p<0.001.
Values lower than 0.3 were suppressed except in item A9.
Factor A (daily functioning domain) explained 10.9% of total variance and covered all items. The Factor B (social interactions domain) explained 8.03% of total variance and covered almost items excepted for B8 (need to be left alone) and B12 (inadequate and/or infrequent sexual intimacy). These items were included in Factor C. The Factor C (emotional functioning domain) explained 17.82% of total variance and covered all items. Factor D (symptoms domain) covered all five symptoms items and explained 12.49% of variance.
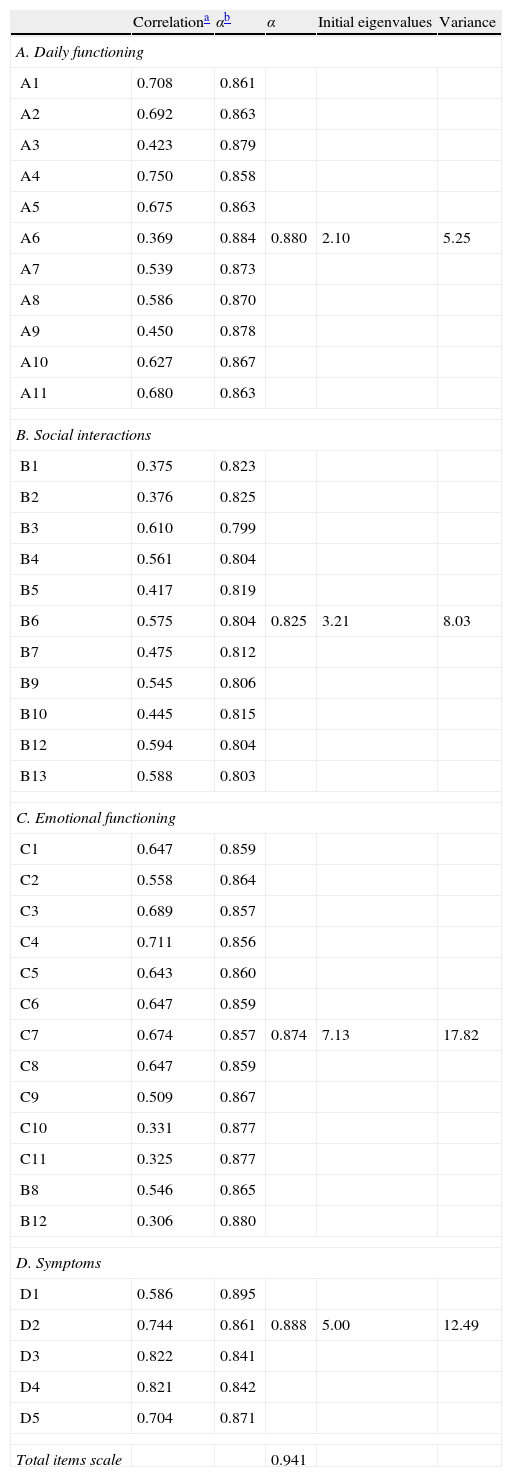
Cronbach's alpha coefficients of internal reliability were above the standard (0.70) for all domains: daily functioning 0.88; social interaction 0.84; emotional functioning 0.87; and symptoms 0.89. Cronbach alpha for total SAQLI was 0.94 with item correlations ranging from 0.30 to 0.70 (Table 3).
SAQLI Cronbach alpha for each domain and the total scale.
| Correlationa | αb | α | Initial eigenvalues | Variance | |
| A. Daily functioning | |||||
| A1 | 0.708 | 0.861 | |||
| A2 | 0.692 | 0.863 | |||
| A3 | 0.423 | 0.879 | |||
| A4 | 0.750 | 0.858 | |||
| A5 | 0.675 | 0.863 | |||
| A6 | 0.369 | 0.884 | 0.880 | 2.10 | 5.25 |
| A7 | 0.539 | 0.873 | |||
| A8 | 0.586 | 0.870 | |||
| A9 | 0.450 | 0.878 | |||
| A10 | 0.627 | 0.867 | |||
| A11 | 0.680 | 0.863 | |||
| B. Social interactions | |||||
| B1 | 0.375 | 0.823 | |||
| B2 | 0.376 | 0.825 | |||
| B3 | 0.610 | 0.799 | |||
| B4 | 0.561 | 0.804 | |||
| B5 | 0.417 | 0.819 | |||
| B6 | 0.575 | 0.804 | 0.825 | 3.21 | 8.03 |
| B7 | 0.475 | 0.812 | |||
| B9 | 0.545 | 0.806 | |||
| B10 | 0.445 | 0.815 | |||
| B12 | 0.594 | 0.804 | |||
| B13 | 0.588 | 0.803 | |||
| C. Emotional functioning | |||||
| C1 | 0.647 | 0.859 | |||
| C2 | 0.558 | 0.864 | |||
| C3 | 0.689 | 0.857 | |||
| C4 | 0.711 | 0.856 | |||
| C5 | 0.643 | 0.860 | |||
| C6 | 0.647 | 0.859 | |||
| C7 | 0.674 | 0.857 | 0.874 | 7.13 | 17.82 |
| C8 | 0.647 | 0.859 | |||
| C9 | 0.509 | 0.867 | |||
| C10 | 0.331 | 0.877 | |||
| C11 | 0.325 | 0.877 | |||
| B8 | 0.546 | 0.865 | |||
| B12 | 0.306 | 0.880 | |||
| D. Symptoms | |||||
| D1 | 0.586 | 0.895 | |||
| D2 | 0.744 | 0.861 | 0.888 | 5.00 | 12.49 |
| D3 | 0.822 | 0.841 | |||
| D4 | 0.821 | 0.842 | |||
| D5 | 0.704 | 0.871 | |||
| Total items scale | 0.941 | ||||
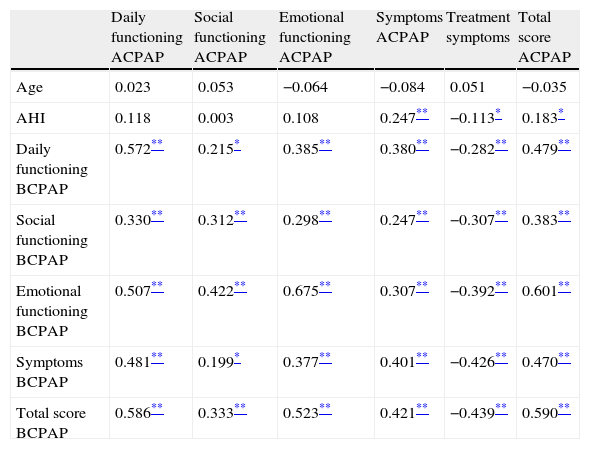
Pearson correlation coefficients between SAQLI interdomains before and after CPAP treatment and clinical variables are presented in Table 4. The four domains before CPAP treatment and total SAQLI after CPAP treatment were highly correlated: daily functioning (r=0.572, p<0.001), social interaction (r=0.312, p<0.001), emotional functioning (r=0.675, p<0.001) and symptoms (r=0.401, p<0.001). Total score of SAQLI is highly correlated before and after CPAP treatment (r=0.590, p<0.001). AHI is highly correlated with Symptom domain (r=0.247, p<0.001) and slightly negative correlated with treatment symptoms (r=−0.113, p<0.005) and positively with total SAQLI score (r=0.183, p<0.005) after CPAP treatment.
Interdomain correlation between SAQLI before and after CPAP treatment.
| Daily functioning ACPAP | Social functioning ACPAP | Emotional functioning ACPAP | Symptoms ACPAP | Treatment symptoms | Total score ACPAP | |
| Age | 0.023 | 0.053 | −0.064 | −0.084 | 0.051 | −0.035 |
| AHI | 0.118 | 0.003 | 0.108 | 0.247** | −0.113* | 0.183* |
| Daily functioning BCPAP | 0.572** | 0.215* | 0.385** | 0.380** | −0.282** | 0.479** |
| Social functioning BCPAP | 0.330** | 0.312** | 0.298** | 0.247** | −0.307** | 0.383** |
| Emotional functioning BCPAP | 0.507** | 0.422** | 0.675** | 0.307** | −0.392** | 0.601** |
| Symptoms BCPAP | 0.481** | 0.199* | 0.377** | 0.401** | −0.426** | 0.470** |
| Total score BCPAP | 0.586** | 0.333** | 0.523** | 0.421** | −0.439** | 0.590** |
BCPAP – before CPAP Treatment; ACPAP – after CPAP treatment.
To test for external validity, interdomains correlations between SAQLI and HADS were performed. All SAQLI domains were negatively correlated with anxiety and depression scales. The emotional functioning domain of SAQLI had the highest correlation with anxiety (r=−0.657, p<0.001) and depression (r=−0.637, p<0.001); then the daily functioning domain correlated with anxiety (r=−0.450, p<0.001) and depression (r=−0.526, p<0.001); following the social functioning domain correlated with anxiety (r=−0.422, p<0.001) and depression (r=−0.471, p<0.001); finally symptoms domain correlated with anxiety (r=−0.444, p<0.001) and depression (r=−0.416, p<0.001).
Differences before and after CPAP treatmentAfter CPAP treatment there was a significant decrease in somnolence with only 20.9% of patients with Epworth sleepiness scale>10.
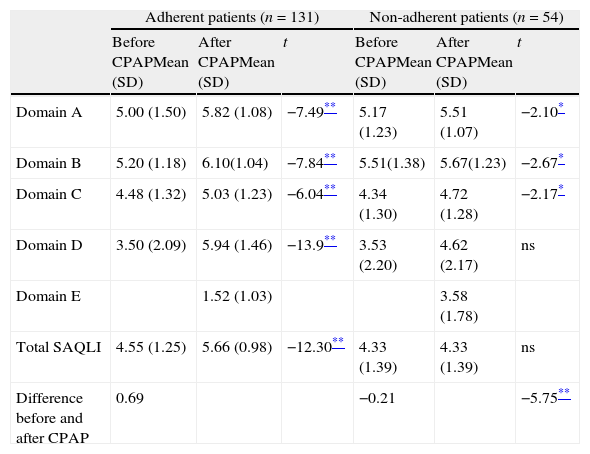
According to SAQLI formula (multiplication of weighting factor by the mean score for treatment related symptoms), a minimum important difference superior to 0.5 was found in the adherent group before and after CPAP therapy, suggesting a change in quality of life. That did not occur in the non-adherent patients. A significant difference was obtained in each domain before and after treatment intervention and between total scores of SAQLI in both moments, in the adherent patients. In non-adherent patients, slightly significant differences were obtained for each domain of quality of life, but not for total quality of life. Table 5 shows the mean and standard deviation, evaluated before and after CPAP therapy and the differences between two moments for both adherent and non-adherent patients.
Differences between SAQLI domains and clinical evaluated before and after CPAP therapy (adherent and non-adherent patients).
| Adherent patients (n=131) | Non-adherent patients (n=54) | |||||
| Before CPAPMean (SD) | After CPAPMean (SD) | t | Before CPAPMean (SD) | After CPAPMean (SD) | t | |
| Domain A | 5.00 (1.50) | 5.82 (1.08) | −7.49** | 5.17 (1.23) | 5.51 (1.07) | −2.10* |
| Domain B | 5.20 (1.18) | 6.10(1.04) | −7.84** | 5.51(1.38) | 5.67(1.23) | −2.67* |
| Domain C | 4.48 (1.32) | 5.03 (1.23) | −6.04** | 4.34 (1.30) | 4.72 (1.28) | −2.17* |
| Domain D | 3.50 (2.09) | 5.94 (1.46) | −13.9** | 3.53 (2.20) | 4.62 (2.17) | ns |
| Domain E | 1.52 (1.03) | 3.58 (1.78) | ||||
| Total SAQLI | 4.55 (1.25) | 5.66 (0.98) | −12.30** | 4.33 (1.39) | 4.33 (1.39) | ns |
| Difference before and after CPAP | 0.69 | −0.21 | −5.75** | |||
ns – non significative.
Researchers and clinicians increasingly recognize the importance of the construct, quality of life. However, there seems to be some confusion on how to measure quality of life and how to use it in clinical practice.16 More precisely, as Flemons and Reimer15 pointed out, the development of a specific measure should be preceded by the identification of the areas of quality of life which are impaired by a specific disease, in this case OSAS. So it is extremely important to assess OSAS impairment with a valid and responsive measure like SAQLI. The present study is the first to explore the factor structure in Western countries. To the best of our knowledge, there is only one other study that performed a factor analysis in a Chinese version of SAQLI.34 In the original studies, published by Flemons and Reimer,15,20 the initial testing of SAQLI was limited to the examination of its discriminative properties, before and after CPAP treatment, but in a small sample size. In the present study there was big enough number of participants to perform the factor analysis.35
According to results, in the daily functioning domain (Factor A), item A9 appeared to contribute little to the daily functioning domain with a factor loading of less than 0.30 (0.281) but was maintained because it was close to 0.30 and its saturation was much lower in the other domains. This result is similar to what happened in Mok et al. factors’ loadings.34 It seems that this item is redundant in several domains. The items B8 (need to be left alone) and B12 (inadequate and/or infrequent sexual intimacy) in the social interactions domain were mixed together with items of the emotional domain. The same result was obtained in the Chinese version of SAQLI.34 In fact, the authors pointed out that some items in the social interactions domain also explored personal feelings, and social interactions, on the other hand, are affected by emotional states. This overlap suggests that there could be a short version as Lacasse et al. suggested.27 Items in the emotional domain and symptoms domain are presented like in the original version.
The Portuguese version of SAQLI demonstrated excellent internal consistency and similar findings were also obtained in previous Chinese,34 Lithuanian28 and Canadian version.27
An interdomain correlation analysis between SAQLI before and after CPAP treatment was also performed. The responsiveness of the Portuguese version of SAQLI was calculated as the mean change of scores before and after CPAP treatment. Like in the Chinese and Canadian SAQLI versions, the mean scores in all domains significantly increased after CPAP treatment. These significant correlations between domains may emphasize the utility of using SAQLI in two different moments for assessing quality of life in OSAS patients receiving a specific treatment. The use of adapted instruments in different countries may have some advantages particularly the opportunity to compare the levels of quality of life impairment of OSAS patients.
An interesting finding was the significant correlation between AHI (mean score revealing severe OSAS) before treatment and total SAQLI score suggesting an effective change in quality of life after appropriate therapy.28 As SAQLI is a disease specific quality of life instrument it is more responsive to important small changes34 and this fact may explain the significant difference in domain scores before and after CPAP treatment and the minimally important difference rating scores, even among non-adherent patients.
In the present study, it is important to point out the absence of test-retest analysis and construct validity performed with another general quality of life questionnaire. The reason for this option had to do with the extensive protocol that patients had to follow as part of their psychological assessment which precluded the inclusion of another instrument but in future studies, this goal will be pursued.
ConclusionThe Portuguese version of SAQLI demonstrated good content and construct validity and high internal consistency capturing the differences before and after CPAP treatment. As a result, the Portuguese version of SAQLI can be a useful quality of life measure in clinical trials on Portuguese OSAS patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors want to thank Prof. Ward Flemons for granting authorization to adapt SAQLI.
Please cite this article as: Sampaio RS, et al. Adaptação portuguesa do questionário de qualidade de vida nos doentes com síndrome de apneia obstrutiva do sono (SAQLI). Rev Port Pneumol. 2012. http://dx.doi.org/10.1016/j.rppneu.2012.02.009.