Due to the inadequate response to inhaled corticosteroids, patients with difficult-to-control asthma (DCA) are submitted to oral corticosteroids or use of omalizumab. Although it is necessary to treat these patients, a significant relationship between steroid usage and both peripheral and respiratory weakness muscle, results in implications, such as loss of quality of life and compromised lung function. Nonetheless, it is not known whether these patients suffer neurophysiological changes due to drug effect.
ObjectiveTo investigate the neurophysiological and functional characteristics of patients with DCA in order to gain a better understanding of the condition.
MethodA cross-sectional study was carried out involving three groups of patients: DCA-C (use of oral corticosteroids), DCA-O (use of omalizumab) and CG (healthy controls matched for age). The assessment involved the six-minute walk test, sit-to-stand test, static balance on a pressure platform, patellar and Achilles reflexes and quadriceps strength in the dominant leg.
ResultsThe results revealed no statistically significant differences between the control group and DCA groups in relation to neurophysiological aspects. However, the DCA groups exhibited a significant reduction in functional capacity [decreased muscle strength (p<0.05), shorter distance covered on walk test (p<0.05) and lesser number of repetitions on sit-to-stand test (p<0.05)] in comparison to the control group.
ConclusionIndividuals with DCA exhibited a reduction in functional capacity. The DCA-C group also demonstrated a reduction in muscle strength when compared with control group, likely caused by the continual use of corticosteroids. However, no neurophysiological alterations were found in the studied population.
Devido à inadequada resposta aos corticóides inalatórios, os pacientes com asma de difícil controlo (ADC) são submetidos a corticóides orais ou uso de omalizumabe. Embora sejam necessários para o tratamento desses pacientes, há uma relação significativa entre o uso de esteróides e fraqueza muscular periférica e respiratória, resultando em implicações como a perda da qualidade de vida e função pulmonar comprometida. No entanto, não se sabe se estes pacientes sofrem alterações neurofisiológicas devido ao efeito da droga.
ObjetivoInvestigar as características neurofisiológicas e funcionais de pacientes com ADC, para melhor compreensão da doença.
MétodoFoi realizado um estudo transversal em 3 grupos de pacientes: ADCC (que fazem uso de corticóide por via oral); ADCO (que fazem uso do Omalizumab) e GC (controlo saudável da mesma idade). A avaliação foi composta pela prova da marcha dos 6 minutos, teste senta-levanta, equilíbrio estático com a plataforma de pressão, reflexos monossimpáticos (patelar e aquileu) e a força do quadríceps do membro inferior dominante.
ResultadosEmbora os resultados em relação aos aspetos neurofisiológicos mostrem não haver diferença estatisticamente significativa entre os grupos controlo e com ADC, deve ser observado que o grupo com ADC, apresentou importante redução da capacidade funcional, quando comparado ao grupo controlo (diminuição da força muscular (p<0,05), diminuição da distância percorrida no teste da caminhada (p<0,05) e diminuição do número de repetições no teste senta/levanta (p<0,05).
ConclusãoIndivíduos com ADC apresentaram redução da capacidade funcional. O grupo ADCC também demonstrou redução na força muscular quando comparado ao grupo controlo, provavelmente causado pelo uso contínuo de corticóides. No entanto, nenhuma alteração neurofisiológica foi encontrada na população estudada.
Asthma is a chronic inflammatory disease characterized by hyper-sensitivity of the lower airways and a variable degree of airflow limitation.1–3 Patients generally respond to treatment with inhaled corticosteroids (with or without the addition of long-acting beta agonists or other medications), with a reduction in inflammation, obstruction and hyper-sensitivity of the airways. However, complete reversibility of the symptoms does not occur in 5–10% of patients. Severe asthma or difficult-to-control asthma (DCA) is the term employed when asthma is insufficiently controlled for more than six months despite adequate treatment tailored to the level of clinical severity, as indicated by a specialist.4–6
Due to the inadequate response to inhaled corticosteroids, patients with DCA are submitted to oral (systemic) corticosteroids, where the main action is the inhibition of recruitment of inflammatory cells and inhibition of release of pro-inflammatory mediators and cytokines from activated inflammatory and airway epithelial cells.6 Another option is the use of omalizumab, an anti-IgE antibody, which works by preventing the attachment of IgE to high affinity receptor thereby avoiding activation of mast cells and basophils, from the initial phase of the allergic response, and blocks the attachment of immunoglobulin to the existing low-affinity receptor in B lymphocytes and in several types celulares.7–12
Although it is necessary for treatment of these patients, a significant relationship between steroid usage and both peripheral and respiratory muscle weakness has been reported in chronic pulmonary disease13 and cystic fibrosis,14 because, in skeletal muscle, corticosteroids decrease the rate of protein synthesis and increase the rate of protein breakdown contributing to atrophy.15–18
The resulting weakness of peripheral and respiratory muscles may have major clinical implications, such as loss of quality of life, fatigue, impaired wound healing, compromised lung function, and poor immune response. However, it is not known whether these patients suffer neurophysiological changes due to drug effect, but to understand this, it is important to investigate the neurological and functional alterations in individuals with DCA with use of the corticosteroids or omalizumab in order to establish a better directed therapeutic intervention.
The aim of the present study, therefore, was to investigate the neurophysiological and functional characteristics of patients with DCA in order to get a better understanding of the condition and monitoring processes in the pulmonary rehabilitation of such individuals.
Materials and methodsPatientsA cross-sectional study was carried out at the Biodynamics of Human Movement Laboratory. The patients were recruited from the Difficult-to-Control Asthma Clinic.
The inclusion criteria were as follows: age between 40 and 60 years; diagnosis of DCA; history of daily symptoms (cough, dyspnea, tightness in chest and wheezing); nocturnal awakening; continuous limitation of activities; frequent exacerbation; FEV1≤80% of best value exhibited by patient; and symptoms controlled or partially controlled at time of evaluation. The exclusion criteria were regular physical exercise (three or more times a week); smoking; uncontrolled DCA; and participation in lung rehabilitation program.
The patients were divided into two groups: those who used an oral corticosteroid (DCA-C; 12 individuals) and those who used omalizumab (DCA-O; 6 individuals). A third group made up of 11 healthy subjects paired for age served as the control group (CG); the inclusion criteria for the CG were the absence of pulmonary disease, no current or past smoking habits and no regular physical exercise that were assessed with the use of the International Physical Activity Questionnaire-short form (IPAQ), and subjects recruited were classified as insufficiently active (does not perform any physical activity, or performs physical activity but not enough to be classified as of moderate or high intensity).
All participants signed a term of informed consent and were told they could withdraw from the study at any stage without being penalized in any way. The study was approved by the Human Research Ethics Committee of the (process no. 334/09). At the end of the informative phase (term of informed consent), guidance was given about the activities that would take place during the data acquisition phase and the objectives of the study.
ProceduresElectromyographic evaluationThe components of the signal acquisition system (electrodes and load cell) were connected to a signal conditioning module, in which the analog signals amplified tenfold were amplified a second time with a 100-fold gain, totaling a final gain of 1000, and filtered with a 10–500Hz bandpass filter. Two pairs of bipolar, differential, active surface electrodes with an 80dB common mode rejection ratio were placed on the motor point of the rectus femoris (RF) and soleus (SO) muscles, according to the guidelines of the SENIAM Project.19
The electromyographic signal was captured during the reflex test performed with a neurological exam hammer adapted with a switch on the area of percussion. For the patellar reflex, the patient remained seated with legs dangling and the patellar tendon was struck with a short, fast blow, while the extension of the knee was observed. For the Achilles reflex, the patient remained in ventral decubitus, with feet dangling off the table and the Achilles tendon slighted tensed by discreet passive dorsiflexion of the foot; the tendon was struck, inducing involuntary plantar flexion20.
Assessment of muscle strengthThe seated patient performed extension of the knee under resistance at maximal strength capacity–peak strength of isometric contraction of the dominant quadriceps. The data were collected using a load cell (kg/force) coupled to the electromyographic signal conditioning module.21
Assessment of static balanceA force plate (TekScan, MatScan model) measuring 0.50cm×0.60cm was used, on which oscillations of the force points in relation to velocity and anteroposterior and laterolateral displacement were analyzed, allowing the assessment of balance based on the oscillating center of force, which is the result of these two variables. This measurement system contains 2288 force sensors arranged in rows and columns on the platform and connected to a data acquisition system controlled by the Research Foot 5.60 program (TekScan) for data storage and analysis by the computer.
The participant was instructed to remain in a static position on the platform with feet spaced at shoulder width. After the calibration of the system based on body weight, the participant remained in biped position for 60s with head aligned, concentrating on a fixed point on the wall at eye height without talking.22
Sit-to-stand testFor the sit-to-stand test, a standard chair (height: 46cm) with no arms was used. After a demonstration, the participants were instructed to stand up from the chair and sit back down without using their hands, repeating the procedure as many times as possible in one minute at a velocity in which the participants felt safe and comfortable. The number of repetitions was recorded.23
Six-minute walk testThe six-minute walk test was performed in a corridor 30m in length and free from pedestrian traffic. The participants were instructed to walk as fast as possible in a six-minute period. During the test, standardized words of encouragement were given each minute. The distance traveled was recorded.24
Statistical analysisThe Shapiro–Wilk test determined normal distribution for all data. Thus, one-way ANOVA was used for the comparison of mean values between the three groups and Tukey's DHS test was used for multiple means in the presence of significance. The level of significance was set at 5%.
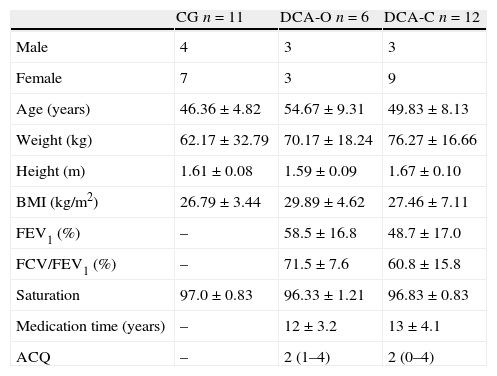
ResultsForty individuals with DCA were recruited, eleven were excluded as not meeting the inclusion criteria, three were excluded because of associated respiratory diseases, four were excluded because they were hospitalized and four were excluded as they did not show up for the evaluation. Thus, the total sample with DCA was made up of 18 individuals. Twenty-nine individuals participated in the present study, distributed among three different groups: CG (n=11), DCA-C (n=12) and DCA-O (n=6). No statistically significant differences were detected between groups with regard to age, weight, height or body mass index (p>0.05), thereby demonstrating the homogeneity of the sample (Table 1).
Anthropometric characteristics of sample.
| CG n=11 | DCA-O n=6 | DCA-C n=12 | |
| Male | 4 | 3 | 3 |
| Female | 7 | 3 | 9 |
| Age (years) | 46.36±4.82 | 54.67±9.31 | 49.83±8.13 |
| Weight (kg) | 62.17±32.79 | 70.17±18.24 | 76.27±16.66 |
| Height (m) | 1.61±0.08 | 1.59±0.09 | 1.67±0.10 |
| BMI (kg/m2) | 26.79±3.44 | 29.89±4.62 | 27.46±7.11 |
| FEV1 (%) | – | 58.5±16.8 | 48.7±17.0 |
| FCV/FEV1 (%) | – | 71.5±7.6 | 60.8±15.8 |
| Saturation | 97.0±0.83 | 96.33±1.21 | 96.83±0.83 |
| Medication time (years) | – | 12±3.2 | 13±4.1 |
| ACQ | – | 2 (1–4) | 2 (0–4) |
CG: control group; DCA-O: difficult-to-control asthma with use of omalizumab; DCA-C: difficult-to-control asthma with use of oral corticosteroid; BMI: body mass index; FEV1: forced expiratory volume in first second; ACQ: asthma control questionnaire. One-way ANOVA and Tukey's post hoc test: *p<0.05.
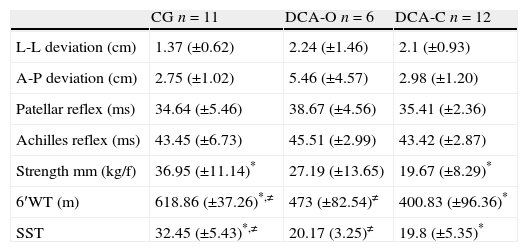
No statistically significant differences were detected between groups with regard to neurophysiological aspects (p>0.05). The CG differed significantly from the DCA groups with regard to functional capacity, achieving a greater number of repetitions on the sit-to-stand test and a longer distance on the six-minute walk test. Regarding muscle strength, as measured by the analysis of the quadriceps muscle, the CG only differed from the DCA-C (Table 2).
Mean and standard deviation values referring to neurophysiological and functional capacity assessments.
| CG n=11 | DCA-O n=6 | DCA-C n=12 | |
| L-L deviation (cm) | 1.37 (±0.62) | 2.24 (±1.46) | 2.1 (±0.93) |
| A-P deviation (cm) | 2.75 (±1.02) | 5.46 (±4.57) | 2.98 (±1.20) |
| Patellar reflex (ms) | 34.64 (±5.46) | 38.67 (±4.56) | 35.41 (±2.36) |
| Achilles reflex (ms) | 43.45 (±6.73) | 45.51 (±2.99) | 43.42 (±2.87) |
| Strength mm (kg/f) | 36.95 (±11.14)* | 27.19 (±13.65) | 19.67 (±8.29)* |
| 6′WT (m) | 618.86 (±37.26)*,≠ | 473 (±82.54)≠ | 400.83 (±96.36)* |
| SST | 32.45 (±5.43)*,≠ | 20.17 (3.25)≠ | 19.8 (±5.35)* |
CG: control group; DCA-O: difficult-to-control asthma with use of omalizumab; DCA-C: difficult-to-control asthma with use of oral corticosteroid; L-L: laterolateral; A-P: anteroposterior; Strength mm: muscle strength of quadriceps; 6′WT (m): six-minute walk test in meters; SST: sit-to-stand test (number of repetitions). One-way ANOVA and Tukey's post hoc test: *=difference between CG and DCA-C (p<0.05). ≠=difference between CG and DCA-O (p<0.05).
The results revealed that the group that used the oral corticosteroid (DCA-C) had the lowest FEV1, achieved the shortest distance on the six-minute walk test and achieved the smallest number of repetitions in the sit-to-stand test, thereby suggesting muscle weakness, which was confirmed by the assessment of quadriceps muscle strength. However, despite exhibiting greater obstructive severity and lower functional capacity, the DCA-C group was similar to the DCA-O and control groups with regard to neurophysiological aspects.
Supplemental oxygen was not needed during the evaluations, as the patients did not exhibit a drop in SpO2 greater than 4% of the baseline condition or clinical signs that indicated oxygen use, thereby avoiding masking the results obtained.
No statistically significant differences between groups were found regarding static balance, as assessed by oscillations from the center of pressure on a force plate. There were also no statistically significant differences between groups regarding latency time in the patellar and Achilles reflexes. However, patients with chronic obstructive pulmonary disease (COPD) were found to have motor impairment, reduced reflex response, muscle weakness and lower functional capacity in comparison to healthy subjects, probably due to the sensory-motor impairment caused by hypoxemia.16,25,26 Kayacan et al.26 report neurophysiological alterations in 93.8% of patients with COPD and Jann et al.27 report a slight reduction in nerve conduction velocity and a reduction in the amplitude of the action potential of motor units in cases of chronic respiratory failure, suggesting the occurrence of peripheral neuropathy. Although there is evidence that patients with DCA may exhibit physical limitations, this was not true of this study, where no alterations of neurophysiological in these individuals were found.
Ozalevli et al.23 compared the sit-to-stand test with the six-minute walk test and found that the former can determine functional capacity equally well as the latter, with less hemodynamic stress in patients with lung conditions. According to the authors, the sit-to-stand test was also correlated with dyspnea and peripheral muscle strength. The present study corroborates these findings.
Muscle strength differed significantly between the DCA-C and control groups, probably due to fact that the continual use of corticosteroids causes myopathy.15–18 This finding does not corroborate results reported in a study carried out by Bruin,28 which found that asthmatic patients (mild and moderate) demonstrated no alterations in cross-section area during peak muscle strength generated by the quadriceps in comparison to healthy subjects. Nevertheless, Lim29 described that prolonged treatment with corticosteroids resulted in important muscle weakness of the quadriceps and respiratory muscle force in patients with asthma and COPD.
Although all patients with DCA demonstrated a reduction in functional capacity, the group treated with omalizumab (DCA-O) did not exhibit a reduction in muscle strength in comparison to the control group. It should be stressed that patients using omalizumab are not susceptible to the diverse side effects of corticosteroids, including skin problems, diabetes mellitus, systemic arterial hypertension, heart failure, osteoporosis, etc., which leads one to infer that patients who make use of omalizumab appear to benefit more. The disadvantage of omalizumab is its high cost (approximately € 815/month), which means that even when offered by the Brazilian public healthcare system, this medication is not freely released and is only distributed in specific centers.
While no statistically significant differences were detected between groups with regard to neurophysiological aspects, all patients with DCA exhibited a reduction in functional capacity in comparison to the control group (reduction in muscle strength, shorter distance traveled on six-minute walk test and fewer repetitions on sit-to-stand test).
ConclusionIndividuals with DCA exhibited a reduction in functional capacity. The DCA-C group also demonstrated a reduction in muscle strength when compared with the control group, the likely cause being the continual use of corticosteroids. However, no neurophysiological alterations were found in the population studied. These data contribute toward a better understanding of the physical condition of such patients and can be used to improve lung rehabilitation programs for individuals with difficult-to-control asthma.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Ferrari Corrêa JC, et al. Avaliação neurofisiológica e funcional em pacientes com asma de dificil controle. Rev Port Pneumol. 2012. http://dx.doi.org/10.1016/j.rppneu.2012.02.008