To evaluate if the cancer registry database can be used to monitor treatment effectiveness using nivolumab treatment of non-small cell lung cancer (NSCLC) as an example.
MethodAn observational inception cohort was used, where all registered cases of NSCLC with authorisation to initiate treatment with nivolumab were monitored retrospectively to evaluate disease characteristics and response to prior treatments. Current exposure to nivolumab was prospectively characterised and treatment outcomes classified based on the clinical information registered in the patient medical record. The main outcome measure used to assess treatment effectiveness was overall survival (OS). Secondary outcomes considered were progression free survival (PFS) as a measure of effectiveness and occurrence of Adverse Drug Reaction (ADRs) as a measure of safety. Data were analysed using SPSS, version 24.
ResultsA total of 115 patients received treatment with nivolumab for NSCLC, between November 1st 2015 and July 31st 2016, and were registered in the database. The majority were non-squamous type (n=107). The median OS was 11.4 months {CI95%: 11.1–11.7}, with a 1-year survival of 44%, in line with clinical trial data. Median PFS was 5.4 months {CI95%: 2.8–7.9}. Treatment was discontinued in 82 cases, most frequently due to disease progression. There were 38 cases of ADRs documented in the patient medical chart, 21 of which led to treatment discontinuation.
ConclusionThe analysed data suggest that the cancer registry is a powerful tool to monitor treatment effectiveness, although considerable investment is needed to improve the medical culture of recording treatment exposure, particularly documentation of ADRs.
Lung cancer is one of the leading causes of mortality in Portugal and worldwide.1 The visible improvements in survival achieved during the last decade are likely to be a result of the progressive increase in early detection of the disease. The effectiveness of the smoking ban legislation implemented may have had a role, although the impact has not yet been clearly identified.2
Apart from surgery, treatments are mainly developed to increase length of survival but very few lead to cure. Nonetheless, the search for effective treatments remains a hope for all and is of particular interest given the rising costs of marketed cancer treatments.3 The highest proportion of the costs for the Portuguese Health Care System constantly correspond to the therapeutic groups used in cancer treatment, including biological, immunomodulatory and cytotoxic ones.4 The aforementioned treatment related costs are constantly rising and immunotherapy is not only an important factor in this trend but also an area where patient access is sometimes delayed by the standard procedures implemented. Ensuring patient's early access to medicines has long been a subject of concern for the World Health Organisation (WHO) and for all governments.
Several mechanisms exist to favour early access, such as the use of special utilisation authorisations (SUA). These are normally used when the medicines are still under economic evaluation and before negotiations are finalised. Adaptive pathways are a common process through which patients in Portugal can get access to medicines ahead of full market authorisation,5 namely the early access program that was recently used for Nivolumab. Nivolumab is an immunotherapy drug that modulates the programmed death 1 (PD-1) pathway. The binding of the ligands PD-L1 (prevalent in NSCLC) and PD-L2, expressed by tumour cells, to their receptor PD-1, expressed by activated T cells, leads to a downregulation in the immune response, favouring tumour cell survival. Nivolumab is an antibody against the PD-1 immune-checkpoint-inhibitor, which by disrupting the PD-1 mediated signalling, promotes a normal immune response against tumour cells. Nivolumab was approved on the 19th June 2015 by the European Medicines Agency (EMA) following documentation of improvement in OS in clinical trials in advanced NSCLC patients.6,7 The inclusion criteria for the early access program were: stage IIIB or IV NSCLC who had progressed on previous chemotherapy treatment; poor expected outcome with available treatment options; and expected life expectancy of at least 3 months.
These adaptive mechanisms were set up to create the environment for the different stakeholders involved to make informed decisions on innovative medicines. However, for these conversations to be meaningful, real world data must be available and objectively monitored. The most common sources of data for effectiveness studies are population based registries. In Portugal, until July 2017, there were four regional cancer registries South, North, Centre and Azores Islands and recently the creation of a single national registry has been approved.8 Currently, the ROR-Sul, the cancer registry for Southern Portugal and Madeira Islands, has a thorough collection of data including patient identification, cancer diagnosis, tumour characteristics, detailed lines of treatment and patient vital status. In most institutions, all clinical and medical data is entered into the ROR-Sul database.
Given the political and science-based context highlighted, we suggested that the cancer registry traditionally used to monitor disease incidence and survival, could also be used to monitor response to treatment.
MethodsAn observational inception cohort was used, where all registered cases of lung cancer with authorisation to initiate treatment with nivolumab in the early access programme that took place from November 1st 2015 to July 31st 2016 were monitored retrospectively, retrieving disease characteristics and type and response to prior treatment; and also prospectively to characterise exposure to nivolumab and treatment outcomes.
Data sources: The Portuguese Drug Regulatory Agency, INFARMED (Autoridade Nacional do Medicamento e Produtos de Saúde, I.P.) has a database of SUA granted for nivolumab in the context of the early access programme, which was used to ascertain the theoretical study population. The ROR-Sul database was then used to extract these cases which comprised all granted SUA that were treated according to the regulator's instructions.
Eligibility criteria were those matching the characteristics defined by the Regulatory Agency for early access to medication, i.e. patients with a histologically confirmed diagnosis of non-small cell lung cancer, locally advanced or metastatic. Cases were selected based on the following topographic codes, using the 10th International Classification of Disease for Oncology9: C34.0 Main bronchus; C34.1 Upper lobe, lung; C34.2 Middle lobe, lung; C34.3 Lower lobe, lung; C34.8 Overlapping lesion of lung; C34.9 Lung, NOS. No additional criteria were defined as we intended to study real world exposure to nivolumab. As such, the detailed characteristics of the sample extracted are presented in the results section.
Therapeutic response was classified according to the RECIST criteria,10 based on the information available on the medical records and clinical files.
The main outcome measure used to evaluate treatment effectiveness was OS. To estimate OS, the period considered was from the day of treatment initiation with Nivolumab until death from any cause.
PFS was considered a secondary outcome to evaluate treatment effectiveness. To estimate PFS, the period considered was also from the day of treatment initiation with Nivolumab until disease progression. Clinical and imagiological progressions were both considered. Other outcome measures of interest considered were response to treatment and ADRs documented in the medical record.
Statistical analysis: Data were stored in the ROR-Sul cancer registry database, which was then extracted to Excel and subsequently analysed using SPSS version 24.0 (IBM statistics). Analysis focused on the univariate characterisation of study subjects, disease and treatment characteristics. Sub-analysis focused on different subgroups, such as stage of disease at treatment initiation and histology (squamous versus non-squamous). Survival analysis was used to ascertain the time granted by submitting the patient to therapy, measuring the time from the day of starting treatment with Nivolumab until the event (death or disease progression, respectively for OS and PFS). One-year survival with 95% CI was used for comparison with published clinical trial data.6,7 The primary reference considered focuses on non-squamous carcinomas,6 and as such the analysis was subsequently restricted to such cases for the purposes of comparison.
ResultsSample characteristicsA total of 225 SUA were granted by INFARMED, from a maximal of 250 initially established. These 225 SUE were given to 217 patients in 32 different institutions, both private and public, throughout the country. The average time from submission of request by the institutions’ Pharmacy and Therapeutics Committee until decision by the regulator was 6.5±5.1 days.
The majority of these cases were registered in ROR-Sul database (n=115; 53.0%), although most SUAs (84.3%) which enabled analysis of treatment effectiveness originated from the South region of Portugal.
Most cases were male patients (n=73; 63.5%), with a median age at diagnosis of 62 years, with significant difference between genders [male 64.0, female 58.5 (p=0.017)]. Most cases were stage IV NSCLC at diagnosis (n=63; 54.8%). The majority were adenocarcinomas (n=104; 90.4%), and 49 (42.6%) were confined to the upper lobe of the lung.
Prior to treatment with nivolumab, 51.3% of patients had been treated with radiotherapy (n=59), 31.3% with surgery (n=36) and 100% with chemotherapy (n=115). Most patients were treated with two (n=41; 35.6%), three or more (n=39; 33.9%) prior lines of chemotherapy and the remaining had received only one line of treatment (n=34; 29.6%). In 98 patients (85.2%) nivolumab was given to treat metastatic cancer. As nivolumab is only indicated for metastatic or locally advanced disease, the median time until treatment initiation was determined differently, according to the stage at diagnosis. For stages IIIB and IV, the date of diagnosis was used in this calculation; for stages I, II and IIIA, the date used was that of recurrence and/or progression to stages IIIB or IV. Hence, the median time from diagnosis of advanced disease to initiation of treatment with nivolumab was 1.2 years±1.5 {CI95%: 27 days–7.6 years}.
Median duration of treatment with nivolumab was 5.6±4.5 months, and 33 patients (28.7%) were still undergoing treatment at the time of database closure (May 31st 2017). The dose administered at treatment initiation ranged from 100 to 351mg (M=198.8±44mg) and was administered every 15 days, as approved, in 93% of cases. The completeness of the registry for these two treatment variables (not mandatory in the registry) were respectively 94.8% and 93.0%.
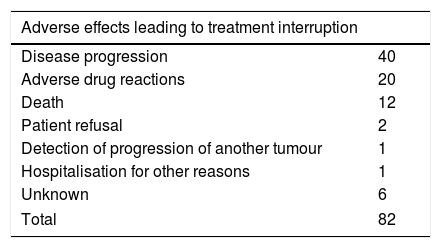
Secondary outcomesOf the 82 patients who stopped treatment, in 76 (92.7%) the reason for treatment withdrawal was documented in the medical record. Most patients discontinued treatment due to disease progression, although a high proportion did so as a result of adverse drug reactions. A total of 38 adverse drug reactions were documented, among which 20 led to treatment discontinuation (Table 1).
Causes of treatment interruption and distribution of adverse drug reactions.
| Adverse effects leading to treatment interruption | ||
|---|---|---|
| Disease progression | 40 | |
| Adverse drug reactions | 20 | |
| Death | 12 | |
| Patient refusal | 2 | |
| Detection of progression of another tumour | 1 | |
| Hospitalisation for other reasons | 1 | |
| Unknown | 6 | |
| Total | 82 | |
| Adverse drug reactions | Leading to treatment interruption | Overall |
|---|---|---|
| Pneumonitis | 4 | 7 |
| Hypothyroidism | 0 | 6 |
| Shortness of breath and respiratory problems | 3 | 4 |
| Gastrointestinal toxicity | 2 | 3 |
| Hematologic toxicity | 2 | 2 |
| Weakness and asthenia | 2 | 2 |
| Metabolic acidosis | 2 | 2 |
| Cutaneous toxicity | 1 | 2 |
| Diarrhoea | 1 | 1 |
| Macular rash | 0 | 1 |
| Hepatic toxicity | 1 | 1 |
| Neurologic toxicity | 1 | 1 |
| Hyperthyroidism | 1 | 1 |
| Diabetes | 0 | 1 |
| Pneumothorax | 0 | 1 |
| Deep vein thrombosis | 0 | 1 |
| Lower limb oedema | 0 | 1 |
| Dermatophytosis and bacterial infection | 0 | 1 |
| Total | 20 | 38 |
Response to treatment with nivolumab was evaluated in all patients: 44 (38.3%) had disease progression, 40 (34.8%) stable disease (34.8%), 18 partial response and one complete response. It was not possible to determine response to treatment for 12 of the patients, ten of whom discontinued treatment.
Disease progression was documented for 70 patients (60.9%). Median PFS was 165 days {CI95%: 83.3–246.7}, equivalent to 5.4 months. The proportion of patients who were progression free at one-year was 36%.
Primary outcomesFrom the 115 patients that started treatment with nivolumab, 56 have died (48.7%). Median OS considering this entire sample was 11.4 months with a one-year OS of 44%. The confidence interval for the median OS was not possible to estimate.
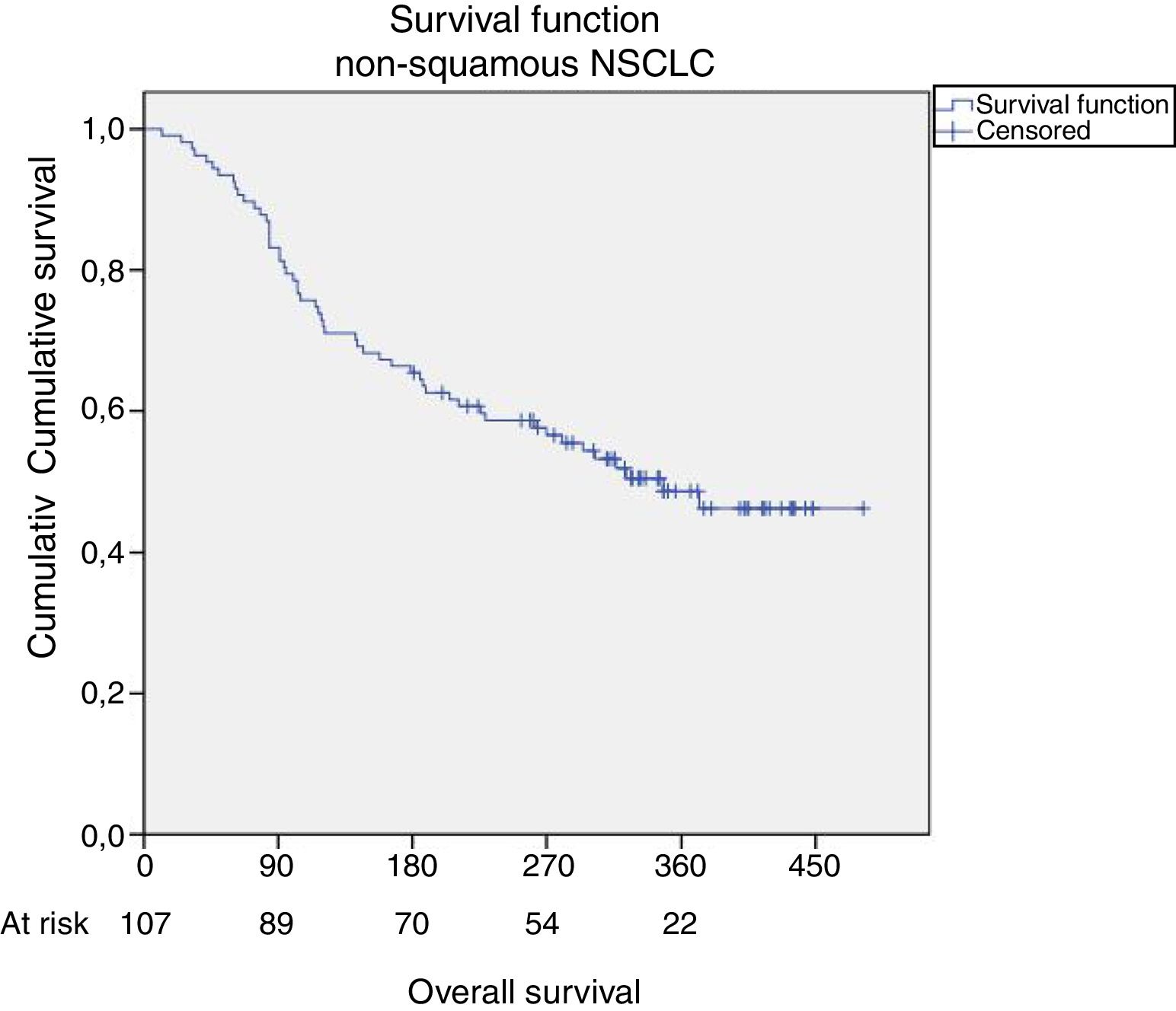
Restricting the analysis to patients with non-squamous carcinomas (n=107), for purposes of comparison with the clinical trials leading to drug approval, there were 53 deaths (49.5%). Median OS in this subgroup was the same, 11.4 months, also with the same one-year OS, 44% (Fig. 1).
Median PFS in the overall sample (n=115) was 5.4 months {CI95%: 2.7–8.1}, slightly reduced when considering the non-squamous subgroup (n=107) to 5.3 months {CI95%: 2.8–7.9}. One-year PFS for the non-squamous subgroup was 36%. In Table 2, the comparison of treatment response is made under comparable conditions to the clinical trial, i.e., only considering non-squamous carcinomas (n=107) and in the case of therapeutic response evaluation, only considering those treated for 9 weeks or over (n=76), a note also indicated in the table footer (Table 2).
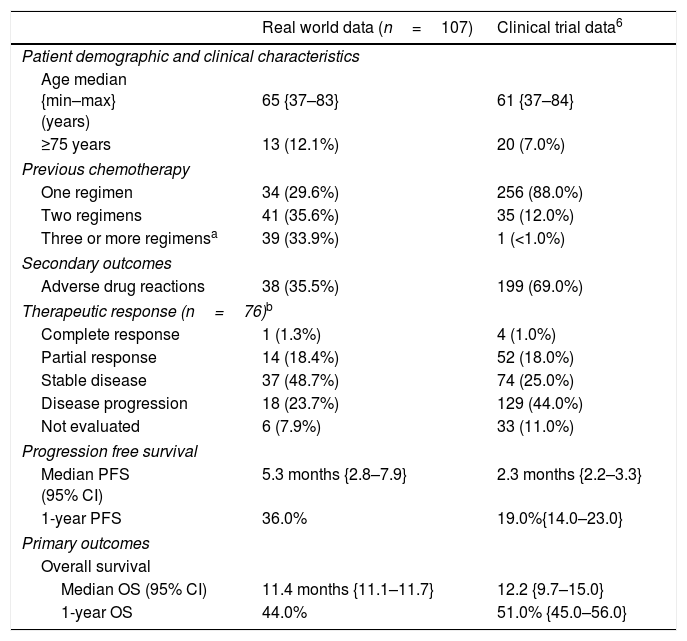
Comparison of clinical trial data with real-world effectiveness data.
| Real world data (n=107) | Clinical trial data6 | |
|---|---|---|
| Patient demographic and clinical characteristics | ||
| Age median {min–max} (years) | 65 {37–83} | 61 {37–84} |
| ≥75 years | 13 (12.1%) | 20 (7.0%) |
| Previous chemotherapy | ||
| One regimen | 34 (29.6%) | 256 (88.0%) |
| Two regimens | 41 (35.6%) | 35 (12.0%) |
| Three or more regimensa | 39 (33.9%) | 1 (<1.0%) |
| Secondary outcomes | ||
| Adverse drug reactions | 38 (35.5%) | 199 (69.0%) |
| Therapeutic response (n=76)b | ||
| Complete response | 1 (1.3%) | 4 (1.0%) |
| Partial response | 14 (18.4%) | 52 (18.0%) |
| Stable disease | 37 (48.7%) | 74 (25.0%) |
| Disease progression | 18 (23.7%) | 129 (44.0%) |
| Not evaluated | 6 (7.9%) | 33 (11.0%) |
| Progression free survival | ||
| Median PFS (95% CI) | 5.3 months {2.8–7.9} | 2.3 months {2.2–3.3} |
| 1-year PFS | 36.0% | 19.0%{14.0–23.0} |
| Primary outcomes | ||
| Overall survival | ||
| Median OS (95% CI) | 11.4 months {11.1–11.7} | 12.2 {9.7–15.0} |
| 1-year OS | 44.0% | 51.0% {45.0–56.0} |
Therapeutic response was compared considering only those patients treated for 9 weeks or longer, as reported elsewhere.6
The clinical outcomes that were observed in this cohort of patients with NSCLC treated with nivolumab were similar to those reported in the clinical trial which had led to its approval in non-squamous NSCLC patients.6 The median OS observed in real world data was 11.4 months, slightly inferior to the 12.2 months reported in the clinical trial but still superior to the 9.4 months reported for treatment with docetaxel. Worth mentioning, we presume that the median OS with docetaxel in real world data would most likely be shorter. Furthermore, the characteristics of patients receiving nivolumab indicate they had worse prognostic factors, with a higher proportion of patients both older than 75 years and having undergone three or more lines of chemotherapy prior to treatment with nivolumab.
It is worth noting that although the observed OS was slightly inferior to that reported in the clinical trial, the PFS was superior (5.3 versus 2.3 months).6 One plausible reason is that in clinical trials, patients are more frequently subject to CTs, hence progressions are detected earlier. In real life, CTs are often delayed by waiting times associated with an overloaded healthcare system. PFS at one-year was higher than in the clinical trial (36.0% versus 19.0%).6 This finding seems surprising and we believe it could be a result of some study limitations, namely the fact that considering disease progression could be a result of observed computerised tomographies (CTs), but also of clinical judgement; also, in real life various oncologists judge disease progression, which could lead to misclassification bias.
We have demonstrated that the cancer Registry database may be successfully used to monitor treatment outcomes, although a great effort needs to be placed on ensuring the exhaustiveness of registered data.
The main limitation of this study is the scant information on patients treated in central and north regions. The new legislation for the National Oncology Registry8 which anticipates mandatory registration of cases, as opposed to the voluntary registration observed at the regional level observed since 1988,11 will certainly be fundamental in overcoming this problem. As a consequence of this limitation, the sample is smaller than ideal, limiting our capacity to undertake potentially interesting sub-analysis. For example, it could have been useful to compare OS of patients undergoing nivolumab as second line treatment compared to those exposed as third, fourth or even fifth line of treatment.
The other limitations of this study derived from sometimes insufficient information in medical records. Oncologists involved in the study had a determinant role in this aspect. A great effort was made to motivate colleagues to document in detail all aspects of treatment. Traditionally, physicians focus on delivering clinical care and, due to time constraints inherent to the health system structure and its demands, minimise documentation in patient medical records. As a result, some variables are more often detailed than others. Tumour characterisation is one example of a set of variables where accuracy is extremely high. In contrast, outside clinical trials, treatment characterisation is less detailed. There needs to be a change in mentality and work organisation in this new era of real life data collection and analysis. Treatment must be seen as an essential component of the registry. To fully characterise drug exposure, it is of utmost importance to know the treatment duration, dose delivered, adverse drug reactions observed and response to treatment.
The proportion of ADRs reported was low. This is probably a result of under registration rather than extreme safety of nivolumab. Therefore, results need to be interpreted with caution. Among registered ADRs, grades were very seldom documented, supporting the previous assumption. We are currently working in the cancer registry on upgrading the fields for monitoring ADRs to enable the quantification of grades and also to document all observed ADRs and not merely the ones leading to treatment discontinuation. This is particularly important because most of these drugs are subject to additional monitoring according to the pharmacovigilance European legislation.
To the best of our knowledge, this is the first national study to show that a population-based cancer registry can be used to monitor the effectiveness of new treatments, providing valuable evidence for policy makers to base reimbursement decisions. In the future, data may be used to determine full or partial reimbursement according to results from different effectiveness profiles. As an example, our data suggests that the benefit obtained using nivolumab in non-squamous cell lung cancers is substantially higher than the benefit obtained in squamous cell cancer (data not shown). Although this result should be interpreted with caution due to the limited sample size of the squamous group, the finding is supported by the published clinical trial data.
ConclusionThe cancer registry is a powerful source of data to monitor the treatment of cancer, provided education, training and, perhaps, incentives are used to change the mentality and the work organisation so that complete treatment data is collected routinely.
FundingThis work received no specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to declare.
To all cancer coordinators, cancer registrars, hospital pharmacists and other allied health care professionals who ensured the exhaustiveness of data registry, from all the hospital units that collaborated in this study.