Fatigue can be divided in perceived fatigue, the feeling of exhaustion or lack of energy, and performance fatigue, the reduction in muscle force/activation during a given task. This meta-analysis evaluates the impact of exercise training on fatigue, compared with normal care in patients with COPD.
Material and MethodsWe searched randomised controlled trials on MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials and CINAHL databases from their inception to December, 31st 2019 using the terms COPD, Fatigue, Fatigability, Muscle activation, Muscle endurance, Muscle Performance, Voluntary Activation, Motoneuron excitability, Force Development, Exercise, AND Rehabilitation.
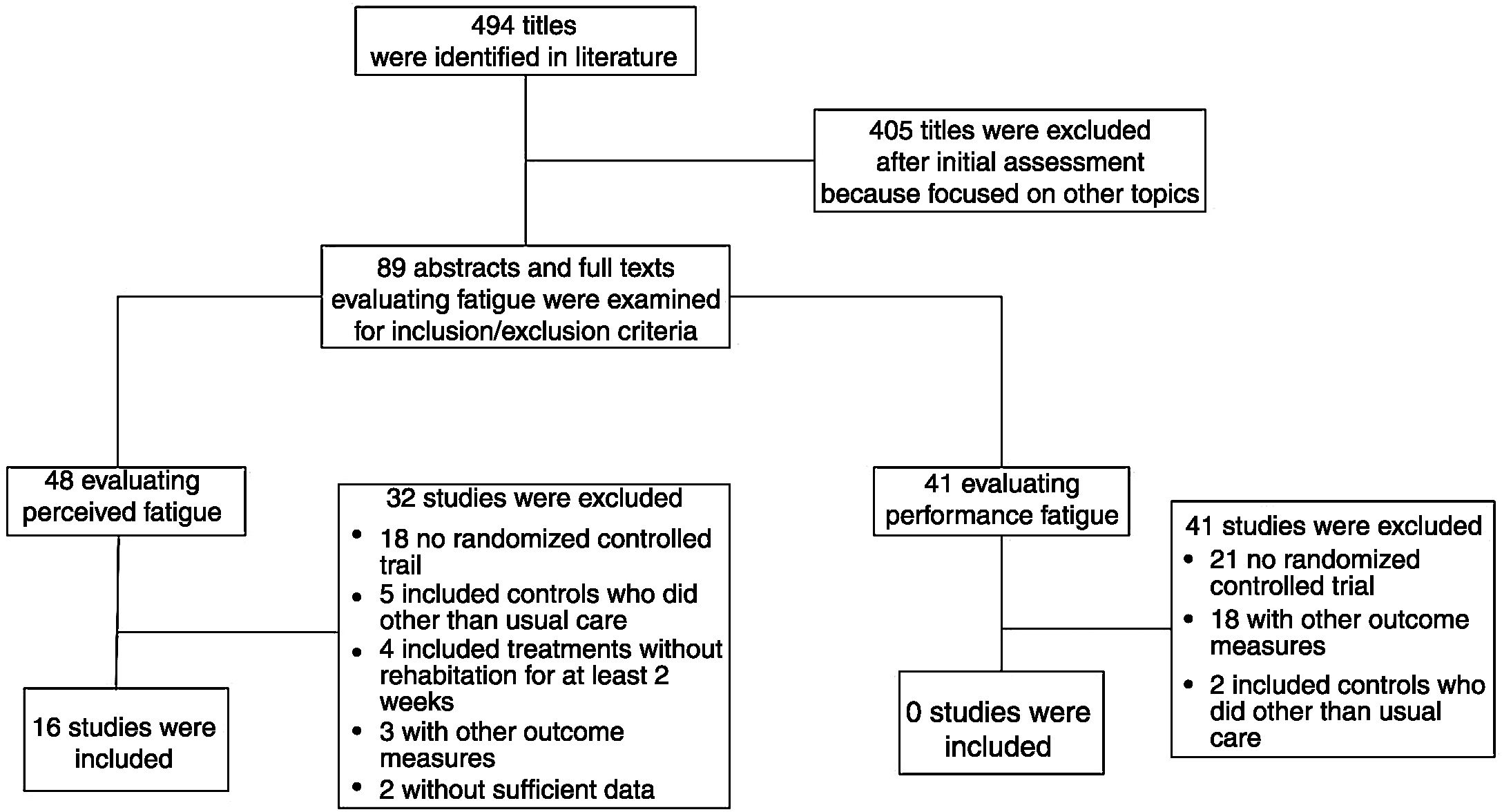
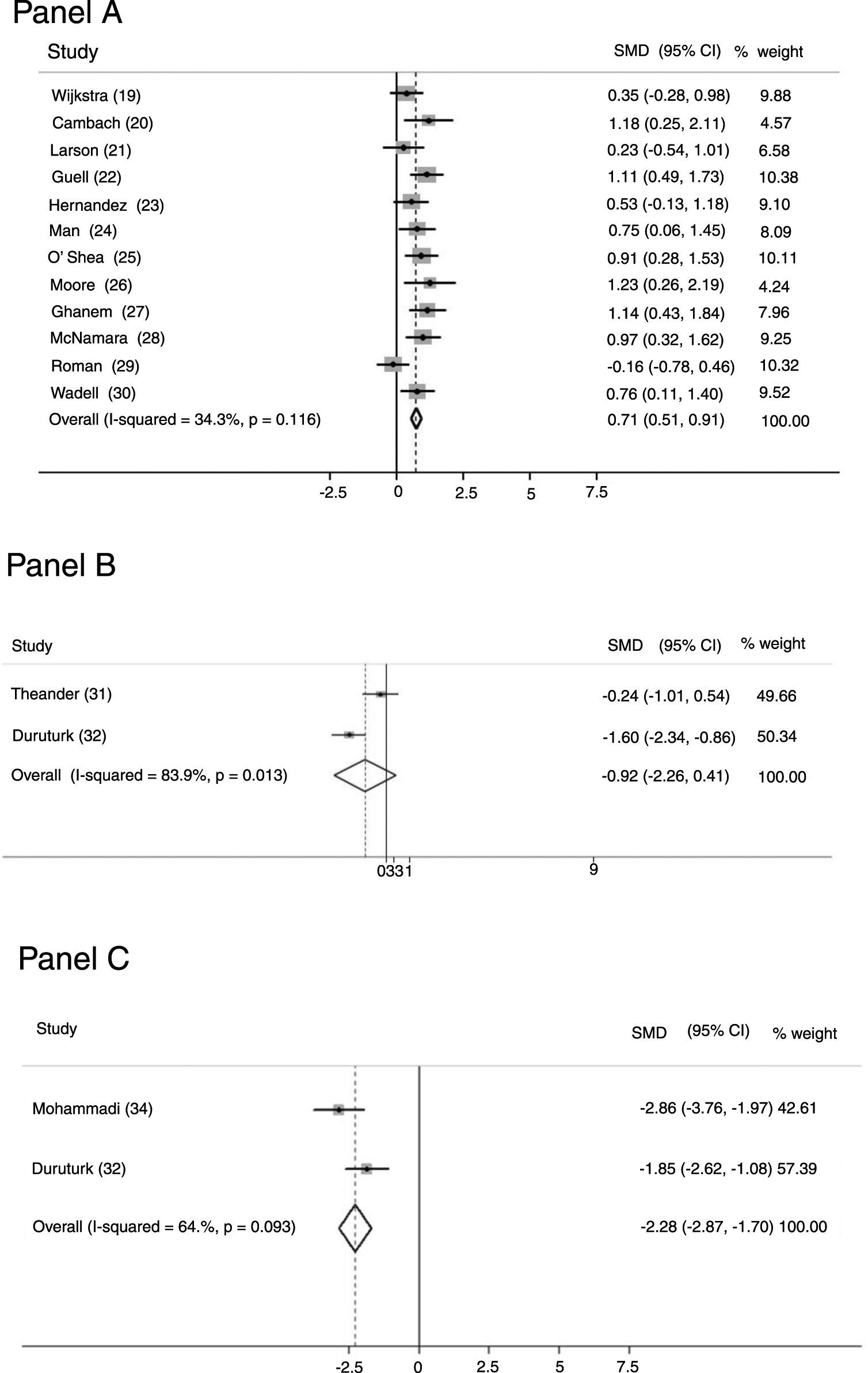
ResultsWe evaluated 494 potential articles. Sixteen, all evaluating perceived fatigability, satisfied the inclusion criteria and were included. Twelve studies (463 patients) assessed fatigue by the Chronic Respiratory Questionnaire showing that intervention improved significantly more than the control group [SMD 0.708; 95% CI 0.510, 0.907; p < 0.001; I² = 34.3%; p = 0.116]. Two studies (68 patients) using the Fatigue Impact Scale, did not find any significant differences between groups [SMD −0.922; 95%CI −2.258, 0.413; p = 0.176; I² = 83.9%; p = 0.013]. Two studies (82 patients) assessed perceived fatigue by the Fatigue Severity Scale: the intervention improved significantly more than the control group [SMD −2.282; 95%CI −2.870, −1.699; p < 0.001; I² = 64.6%, p = 0.093]. No study evaluating performance fatigue was found.
ConclusionsThis study provided low-quality evidence of a positive impact of different exercise training programs on perceived fatigue in patients with COPD. Further studies are needed to assess the effects of exercise training on fatigue and to test tailored programs.
More than half of patients with chronic obstructive pulmonary disease (COPD) experience fatigue which may have a substantial impact on functional impairment, physical activity, health related quality of life (HRQL), mortality, morbidity, hospitalization rate and length of hospital stay.1,2 Lower limb muscles of patients with COPD may show reduced endurance capacity and are more prone to fatigue, due to disuse, the presence of limb muscle dysfunction,3 and exercise vasoconstriction induced by respiratory muscle fatigue.4,5 These mechanisms may be responsible for the onset of the symptom fatigue during daily life.6
It can be divided into perceived fatigue or performance fatigue.7 The first one is a normal response to exercise or stress, a multidimensional perception defined as “the subjective feeling of tiredness, exhaustion or lack of energy, which occurs on a daily basis”.7 However, fatigue may also occur during the performance of a given task, and is defined as performance fatigue: an objective and measurable domain, consisting of the reversible reduction in force generated by the muscles during a given task8 such as a constant load exercise.9 It could be distinguished as central, defined as a progressive reduction in the voluntary activation of muscle during exercise,10 and peripheral fatigability, described by the reduction of muscle activation in or distal to the neuromuscular junction.11
The reduction of fatigue should be one of the aims of comprehensive management of patients with COPD.12 Exercise training is strongly recommended in COPD, being effective in reducing dyspnoea and improving exercise capacity and HRQL.13–15 However, the effects of exercise training on fatigue as a primary aim in patients with COPD have been rarely studied,16,17 and to the best of our knowledge, there is no systematic review of the literature about the effectiveness of exercise training on fatigue in patients with COPD.
Therefore, the aim of this systematic review and meta-analysis of randomized controlled trials (RCTs) in patients with COPD, was to evaluate the impact of interventions including exercise training on perceived and performance fatigue, as compared to ordinary care or education alone.
MethodsThis study conforms to all Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and reports the required information accordingly (Supplementary Checklist, http://links.lww.com/PHM/A364).
Data sources and search strategiesWe searched the following databases: MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, and the Cumulative Index to Nursing and Allied Health Literature, from their inception to December, 31st 2019, with no language restriction. We also reviewed the references of retrieved articles for additional studies. The search was limited only to RCTs using the terms: COPD, Fatigue, Fatigability, Muscle activation, Muscle endurance, Muscle Performance, Voluntary Activation, Motoneuron excitability, Force Development, Exercise, and Rehabilitation.
PatientsWe included RCTs involving patients with COPD according to the Global Initiative for Chronic Obstructive Lung Disease recommendations in all stage of severity.18 We excluded studies involving patients with (1) an acute exacerbation within 4 weeks before starting the intervention; (2) major comorbidities such as chronic heart failure, asthma, and sleep-related disorders.
InterventionsWe included studies administering exercise training programs, with the following characteristics:
- -
Involving in- or out-patients, and home- or community-based programs;
- -
Programs of 2 week minimum duration;
- -
Endurance and/or strength training of lower and/or upper limbs;
- -
Comparing exercise training with usual care, (defined as conventional medical care without any prescription of exercise training or physical activity) and/or education.
We included any study assessing:
- -
Perceived fatigue: by means of scales or questionnaires evaluating subjective perception of fatigue;
- -
Performance fatigue: by means of objective measures evaluating changes in muscle performance after a fatiguing task such as muscle force or electromyography trace during a maximal voluntary contraction (MVC) or stimulated muscle resting Twitch.
Two investigators (LB, MP) independently conducted the search of the databases, screening all titles and/or abstracts for the inclusion criteria. Reviewers then retrieved abstracts and/or full-text papers of all potentially eligible studies and maintained records of all studies not meeting the inclusion criteria. They also provided the reasons for their exclusion.
The investigators inserted the data of potentially eligible articles in a Microsoft Excel (2013 version, Microsoft, Redmond, WA) dedicated database. Disagreement between investigators about eligibility was resolved by discussion and consensus. If consensus could not be reached, a third investigator (MV) adjudicated. All data were checked for accuracy. Missing data were requested by e-mail to the authors. An investigator (LB) retrieved the full-text of the included studies and inserted their data into a dedicated electronic database. Another investigator (MP) independently extracted data from the same studies.
The information collected included the background characteristics of the research reports: characteristics of participants in the study; number of participants who dropped out or withdrew from the study; a full description of the exercise training programs (setting, components, duration, and characteristics); assessments, and associated results. If a study reported multiple group comparisons (e.g., exercise training plus free walking vs exercise training alone vs conventional care), treatment groups performing exercise that could be relevant to outcomes were combined into one virtual intervention group, and this group was compared to the group receiving conventional care.
The investigators assessed papers for bias using the Cochrane Collaboration's tool for assessing risk of bias in RCTs.19 Risk of bias was assessed according to the following domains: sequence generation; allocation concealment; blinding of participants; blinding of personnel; blinding of outcome assessment; incomplete outcome data; selective outcome reporting, and other biases.
Statistical analysisStatistical analysis was performed using STATA version 11.2 software (Stata, College Station, TX). All data were extrapolated from the corresponding full-text studies. For outcome, we recorded mean and standard deviation (SD) of variation from baseline to the end of the study. When SD was not available, we calculated them from standard errors, confidence intervals, or t-values or contacted the trial authors by email for clarification. We excluded studies with data other than mean and SD.20
The standardized mean difference (SMD) was calculated. Heterogeneity of studies was assessed by performing the Q-test considering values of P < 0.01 as significant. The first step of the evaluation was conducted by fixed-effect models using the Mantel-Haenzel method. If a significant heterogeneity among studies was found, a random effect evaluation by the Der-Simonian and Laird method approach was performed.21 Forest plots were used to detect publication bias for meta-analysis evaluation including more than 8 studies.22 For the meta-analysis, only the outcome measures included in at least two studies were analysed.
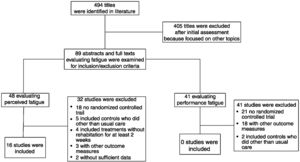
ResultsWe identified 494 potentially relevant articles. Of these, 405 were excluded after the abstracts were read and 89 full-texts were analysed for inclusion criteria. Sixteen out of the 48 studies evaluating perceived fatigue satisfied the inclusion criteria and were included in the meta-analysis23–38 (Fig. 1). No study out of the other 41 evaluating changes in performance fatigue satisfied the inclusion criteria.
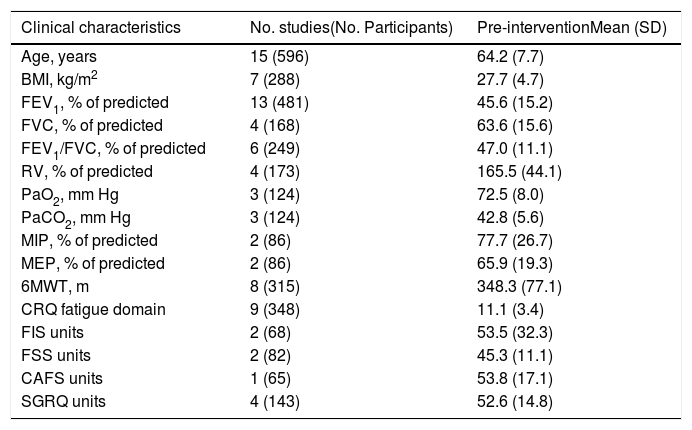
Table 1 shows the baseline characteristics of the included subjects. They suffered from moderate-to-severe COPD. Table 2 shows the main characteristics of all interventions carried out in the studies included.
Demographic, anthropometric and clinical characteristics of included patients.
| Clinical characteristics | No. studies(No. Participants) | Pre-interventionMean (SD) |
|---|---|---|
| Age, years | 15 (596) | 64.2 (7.7) |
| BMI, kg/m2 | 7 (288) | 27.7 (4.7) |
| FEV1, % of predicted | 13 (481) | 45.6 (15.2) |
| FVC, % of predicted | 4 (168) | 63.6 (15.6) |
| FEV1/FVC, % of predicted | 6 (249) | 47.0 (11.1) |
| RV, % of predicted | 4 (173) | 165.5 (44.1) |
| PaO2, mm Hg | 3 (124) | 72.5 (8.0) |
| PaCO2, mm Hg | 3 (124) | 42.8 (5.6) |
| MIP, % of predicted | 2 (86) | 77.7 (26.7) |
| MEP, % of predicted | 2 (86) | 65.9 (19.3) |
| 6MWT, m | 8 (315) | 348.3 (77.1) |
| CRQ fatigue domain | 9 (348) | 11.1 (3.4) |
| FIS units | 2 (68) | 53.5 (32.3) |
| FSS units | 2 (82) | 45.3 (11.1) |
| CAFS units | 1 (65) | 53.8 (17.1) |
| SGRQ units | 4 (143) | 52.6 (14.8) |
BMI, Body Mass Index; FEV1, Forced Expiratory Volume in the 1st second; FVC, Forced Vital Capacity; RV, Residual Volume; MIP, Maximal Inspiratory Pressure; MEP, Maximal Expiratory Pressure; CRQ, Chronic Respiratory Questionnaire; FIS, Fatigue Impact Scale; FSS, Fatigue Severity Scale; CAFS, COPD and Asthma Fatigue Scale; SGRQ, St. George's Respiratory Questionnaire.
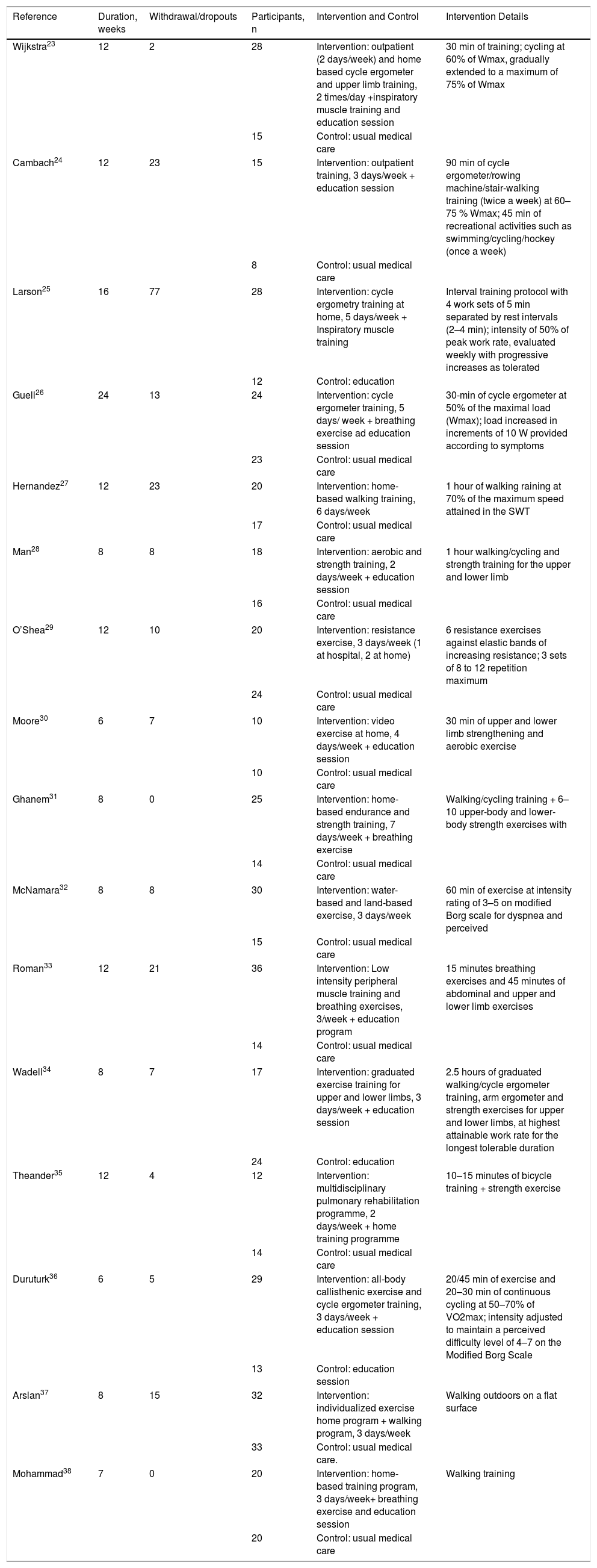
Characteristics of the interventions.
| Reference | Duration, weeks | Withdrawal/dropouts | Participants, n | Intervention and Control | Intervention Details |
|---|---|---|---|---|---|
| Wijkstra23 | 12 | 2 | 28 | Intervention: outpatient (2 days/week) and home based cycle ergometer and upper limb training, 2 times/day +inspiratory muscle training and education session | 30 min of training; cycling at 60% of Wmax, gradually extended to a maximum of 75% of Wmax |
| 15 | Control: usual medical care | ||||
| Cambach24 | 12 | 23 | 15 | Intervention: outpatient training, 3 days/week + education session | 90 min of cycle ergometer/rowing machine/stair-walking training (twice a week) at 60–75 % Wmax; 45 min of recreational activities such as swimming/cycling/hockey (once a week) |
| 8 | Control: usual medical care | ||||
| Larson25 | 16 | 77 | 28 | Intervention: cycle ergometry training at home, 5 days/week + Inspiratory muscle training | Interval training protocol with 4 work sets of 5 min separated by rest intervals (2–4 min); intensity of 50% of peak work rate, evaluated weekly with progressive increases as tolerated |
| 12 | Control: education | ||||
| Guell26 | 24 | 13 | 24 | Intervention: cycle ergometer training, 5 days/ week + breathing exercise ad education session | 30-min of cycle ergometer at 50% of the maximal load (Wmax); load increased in increments of 10 W provided according to symptoms |
| 23 | Control: usual medical care | ||||
| Hernandez27 | 12 | 23 | 20 | Intervention: home-based walking training, 6 days/week | 1 hour of walking raining at 70% of the maximum speed attained in the SWT |
| 17 | Control: usual medical care | ||||
| Man28 | 8 | 8 | 18 | Intervention: aerobic and strength training, 2 days/week + education session | 1 hour walking/cycling and strength training for the upper and lower limb |
| 16 | Control: usual medical care | ||||
| O’Shea29 | 12 | 10 | 20 | Intervention: resistance exercise, 3 days/week (1 at hospital, 2 at home) | 6 resistance exercises against elastic bands of increasing resistance; 3 sets of 8 to 12 repetition maximum |
| 24 | Control: usual medical care | ||||
| Moore30 | 6 | 7 | 10 | Intervention: video exercise at home, 4 days/week + education session | 30 min of upper and lower limb strengthening and aerobic exercise |
| 10 | Control: usual medical care | ||||
| Ghanem31 | 8 | 0 | 25 | Intervention: home-based endurance and strength training, 7 days/week + breathing exercise | Walking/cycling training + 6–10 upper-body and lower-body strength exercises with |
| 14 | Control: usual medical care | ||||
| McNamara32 | 8 | 8 | 30 | Intervention: water-based and land-based exercise, 3 days/week | 60 min of exercise at intensity rating of 3–5 on modified Borg scale for dyspnea and perceived |
| 15 | Control: usual medical care | ||||
| Roman33 | 12 | 21 | 36 | Intervention: Low intensity peripheral muscle training and breathing exercises, 3/week + education program | 15 minutes breathing exercises and 45 minutes of abdominal and upper and lower limb exercises |
| 14 | Control: usual medical care | ||||
| Wadell34 | 8 | 7 | 17 | Intervention: graduated exercise training for upper and lower limbs, 3 days/week + education session | 2.5 hours of graduated walking/cycle ergometer training, arm ergometer and strength exercises for upper and lower limbs, at highest attainable work rate for the longest tolerable duration |
| 24 | Control: education | ||||
| Theander35 | 12 | 4 | 12 | Intervention: multidisciplinary pulmonary rehabilitation programme, 2 days/week + home training programme | 10–15 minutes of bicycle training + strength exercise |
| 14 | Control: usual medical care | ||||
| Duruturk36 | 6 | 5 | 29 | Intervention: all-body callisthenic exercise and cycle ergometer training, 3 days/week + education session | 20/45 min of exercise and 20–30 min of continuous cycling at 50–70% of VO2max; intensity adjusted to maintain a perceived difficulty level of 4–7 on the Modified Borg Scale |
| 13 | Control: education session | ||||
| Arslan37 | 8 | 15 | 32 | Intervention: individualized exercise home program + walking program, 3 days/week | Walking outdoors on a flat surface |
| 33 | Control: usual medical care. | ||||
| Mohammad38 | 7 | 0 | 20 | Intervention: home-based training program, 3 days/week+ breathing exercise and education session | Walking training |
| 20 | Control: usual medical care |
Seven studies24,26,28,32–34,36 were conducted mainly in an out-patient setting, six at home 25,27,30,31,37,38 and three studies23,29,35 in both settings.
SchedulesThe total duration of programs ranged from 6 to 24 weeks. There were two to seven sessions per week. The duration of each session ranged from 30 to 90 min in eleven studies23–28,30,32,33,35,36 and lasted two and a half hours in one study.34 Four studies29,31,37,38 did not report any duration.
InterventionsThirteen studies included exercise training of locomotor muscles: cycling in five studies,23,25,26,35,36 walking in three27,37,38 and a combination of these in five.25,28,31,32,34 In three studies the intervention consisted only of calisthenics exercises.29,30,33
All the studies included endurance training. The intensity ranging from 50% to 75% of either the maximum workload or oxygen consumption (VO2) reached during incremental tests in five studies.23–26,36 McNamara et al.32 used the Borg scale39 to set the intensity of the exercise (from 3 to 5 on modified Borg scale) and Hernandez et al.27 used the 70% of the maximum speed attained in the Incremental Shuttle Walking test (ISWT);40 nine others studies28–31,33–35,37,38 reported no training intensity.
In six studies23,24,26,34,35,36 patients performed continuous training on a cycloergometer, while only one25 used the interval training.
Additional components of exercise trainingIn four studies26,31,34,38 breathing exercises were administered in addition to the exercise program (e.g.: pursued lip breathing and diaphragmatic breathing). In eleven studies23–26,28,30,33–36,38 educational sessions were also administered. Inspiratory muscle training was added in two studies23,25
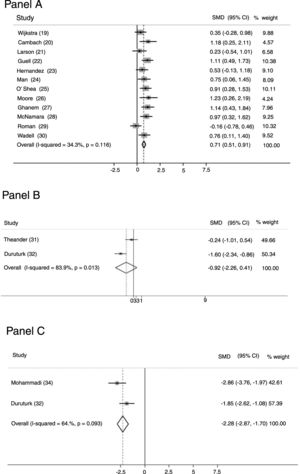
OutcomesTwelve studies23–34 (463 patients: 271 study group and 192 controls) evaluated perceived fatigue by means of the dedicated domain of the chronic respiratory questionnaire (CRQ).41 Only in three studies35,37,38 (18.8%) was fatigue measurement the primary outcome. Fig. 2-Panel A shows the related forest plot: the intervention improved significantly more than the control group [SMD 0.708; 95% CI 0.510, 0.907; p < 0.001; I² = 34.3%; p = 0.116]. Figure 1SM describes funnel plot for studies on fatigue item in CRQ.
Two studies35,36 (68 patients: 41 study group and 27 controls) used the Fatigue Impact Scale (FIS), which examines patients' perceptions of their limitations caused by fatigue on the cognitive, physical, and psychosocial domains.42Fig. 2-Panel B describes the related forest plot: owing to the high heterogeneity in the “fixed analysis model” (P = 0.0001), a random effect model was performed. No differences between intervention and control group were found. [SMD-0.922; 95%CI −2.258, 0.413; p = 0.176; I² = 83.9%; p = 0.013].
Fig. 2-Panel C describes the forest plot for two studies36,38 (82 patients: 49 study group and 33 controls) assessing fatigue by the Fatigue Severity Scale (FSS), a 9-item scale assessing disabling fatigue. Each question evaluates patients’ perception in the form of 7-point modified Likert Scale.43 The intervention group improved significantly more than the control group [SMD -2.282; 95%CI −2.870, −1.699; p < 0.001; I² = 64.6%, p = 0.093]. Only one study37 used the COPD and Asthma Fatigue Scale.44
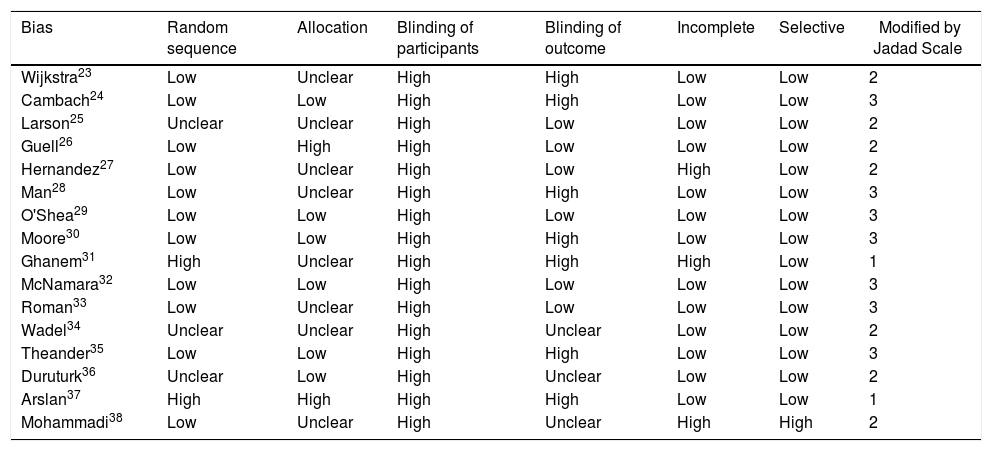
Risk of biasThe assessment of the risk of bias by funnel plots on CRQ revealed high variability in the results of the included studies. Table 3 shows results of the risk of bias assessment. Half of the studies23,25,27,28,31,33,34,38 did not report any detail on randomization and allocation modalities, and there was a high detection bias rate. Almost all studies included showed a high risk of bias for the blinding of participants and personnel.
Risk of bias assessment.
| Bias | Random sequence | Allocation | Blinding of participants | Blinding of outcome | Incomplete | Selective | Modified by Jadad Scale |
|---|---|---|---|---|---|---|---|
| Wijkstra23 | Low | Unclear | High | High | Low | Low | 2 |
| Cambach24 | Low | Low | High | High | Low | Low | 3 |
| Larson25 | Unclear | Unclear | High | Low | Low | Low | 2 |
| Guell26 | Low | High | High | Low | Low | Low | 2 |
| Hernandez27 | Low | Unclear | High | Low | High | Low | 2 |
| Man28 | Low | Unclear | High | High | Low | Low | 3 |
| O'Shea29 | Low | Low | High | Low | Low | Low | 3 |
| Moore30 | Low | Low | High | High | Low | Low | 3 |
| Ghanem31 | High | Unclear | High | High | High | Low | 1 |
| McNamara32 | Low | Low | High | Low | Low | Low | 3 |
| Roman33 | Low | Unclear | High | Low | Low | Low | 3 |
| Wadel34 | Unclear | Unclear | High | Unclear | Low | Low | 2 |
| Theander35 | Low | Low | High | High | Low | Low | 3 |
| Duruturk36 | Unclear | Low | High | Unclear | Low | Low | 2 |
| Arslan37 | High | High | High | High | Low | Low | 1 |
| Mohammadi38 | Low | Unclear | High | Unclear | High | High | 2 |
This systematic review investigated the current evidence of exercise training on fatigue in patients with COPD. The analysis of the 16 RCTs included evaluating the effects on perceived fatigue23–38 showed high heterogeneity of study design, patient sampling, exercise training schedules, and outcome measures assessed. All studies showed a high risk of bias. As a whole, the studies showed low-grade evidence of positive effect of exercise training on perceived fatigue. No study evaluated the effects on performance fatigue.
Fatigue is an important debilitating symptom affecting all chronic respiratory diseases, including COPD. A four-year observational study on fatigue in patients with COPD reported that severe fatigue doubled in patients with mild to severe COPD despite optimal care.45 Fatigue is a leading cause of consultations with major clinical implications. Despite its well-acknowledged negative impact on patient’s life, fatigue is still a misunderstood and underdiagnosed symptom in COPD. As a consequence, there is currently no specific intervention to treat all aspects of this symptom which is quite often considered a secondary outcome in interventions aiming primarily to increase physical fitness and/or HRQL.46
Spruit et al.9 have proposed a model of fatigue in patients with COPD. Moderate to severe fatigue can be the results of systemic, physical, psychological and behavioural factors. Fatigue can be precipitated by infectious COPD exacerbation and its treatment. This model suggests that the fatigue of these patients is not simply the result of COPD and cannot be predicted by the sole degree of airflow obstruction but would be the consequence of multiple factors that may act alone or in interaction, at rest and during/after exercise.10
Rather interestingly in our systematic review, the positive impact of pulmonary rehabilitation on perceived fatigue was found in studies using the CRQ and the FSS but not the FIS. Antoniu and Ungureanu46 identified 8 multidimensional scales which are commonly used to assess fatigue in COPD but 75% of RCTs included in our analysis evaluated fatigue by means of the dedicated domain of the CRQ.41 Houben-Wilke and colleagues recently showed that the item ‘energy’ of the COPD assessment test improves with the greatest effect on size after pulmonary rehabilitation.47 A commonly used multi-dimensional scale to evaluate fatigue is the subjective fatigue subscale of the checklist individual strength (CIS-Fatigue)48 and Peters and colleagues reported a significant mean improvement in CIS-Fatigue score following 12-week of pulmonary rehabilitation in patients with COPD.49 The use of different tools in papers prevents accurate comparisons between studies as the scores produced by the scales show poor to moderate correlations between them.50
It should be noticed that we analysed exercise training, just one (although the main) component of pulmonary rehabilitation which is a comprehensive multidisciplinary intervention including, but not limited to it.13 Therefore the results of our systematic review should not be generalised to pulmonary rehabilitation programs.
On the subject of performance fatigue, it is rather interesting to note that, despite the fact that there are some observational studies describing high prevalence in patients with COPD,51 we were unable to include any RCT of the effects of exercise training on this impairment.
Different tests have been used to evaluate performance fatigue, which depends on the ability of the peripheral muscles and the central nervous system to meet the demands of a prescribed task. Both systems can exhibit abnormal changes in response to exercise and contribute to increased performance fatigue:52,53 we can test it by the changes in stimulated resting Twitch for peripheral involvement and by MVC for the central ones.53 Thereafter, specific methods to train the muscles and reduce the central and/or peripheral component of performance fatigue, such as the maximal strength training54 or neuromuscular electrical stimulation,55 should also be thoroughly investigated in subjects with COPD.
ConclusionThis systematic review has provided low-quality evidence of a positive impact of different exercise training programmes on perceived fatigue in patients with COPD. Further studies with better standardisation and scientific validity are needed to assess the effects of exercise training on fatigue and to test dedicated programs. No study of the efficacy of exercise training on performance fatigue was found.
Authors' contributionsLB, MP and CS contributed to data acquisition; LB, MP and CS contributed to data analysis; MP, MVi, MVe, CS, SS, FS and NA prepared article draft or critically revised it for important intellectual content; all authors gave contribution to conception and design, data interpretation, final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by the "Ricerca Corrente" Funding scheme of the Ministry of Health, Italy. We thank Laura Comini and Adriana Olivares for technical assistance.