Electronic (e-) cigarettes are used to heat liquids producing aerosols for inhalation. Recently there have been reports of a large number of adverse outcomes relating to e-cigarette consumption (vaping), which has been referred to as “vaping associated pulmonary illness” (VAPI).
AimThis review provides an overview of clinical, radiological and pathological features of VAPI in the literature. We also describe a case of VAPI, presenting with symptoms of bronchiolitis, responding well to azithromycin in addition to the usual treatments provided for such cases.
MethodsWe searched original papers, observational studies, case reports, and meta-analyses published between 2000 and 2019 in English in PubMed database using the keywords: e-cigarette, “vaping associated pulmonary illness”, VAPI, EVALI, vaping AND “lung injury”. We also used data of the Centers of Disease Control (CDC) website.
ResultsFrom an initial search of PubMed, 62 potential articles were identified, and another 9 studies were identified from the bibliographies of retrieved articles. In this search we found 7 case series and 16 case reports, which were included in the review. In this search we also found 4 review articles.
ConclusionVAPI is a syndrome presenting with isolated pulmonary or combined pulmonary, gastrointestinal and constitutional symptoms and can be rapidly progressive, leading to respiratory failure, often requiring invasive respiratory support. There is an urgent need for more research on VAPI especially relating to etiology, treatment and prevention.
Electronic cigarette consumption, also called vaping, is done with a hand-held device, powered by a battery, producing aerosols by heating liquids (or e-liquids) containing various components. Compared to the first-generation devices, the second, third and fourth generation devices have higher voltages and frequently include a rechargeable lithium-ion battery. The third and fourth generation devices allow adjustments of voltage and temperature, providing a different type of aerosolization, which can create new compounds.
Aerosols from e-cigarettes. The liquids mainly consist of propylene glycol, glycerin, flavorings and in most cases nicotine. The liquids may also contain tetrahydrocannabinol (THC), the psychoactive component of cannabis. E-cigarette aerosols contain heavy metals and volatile organic compounds. The origin of the heavy metals is assumed to be the metallic coil used to heat the liquid, and also the liquids themselves. With the exception of cadmium, the number of metals is greater than in conventional tobacco cigarettes. Many other constituents of e-cigarette aerosols have been detected, such as acetone, formaldehyde, nitrosonornicotine and tobacco-specific nitrosamine. The long-term health effects of these aerosol components are largely unknown. Other contaminants in e-cigarettes are strongly dependent on different brands of commercially available e-cigarettes or on self-made mixtures with products from the illegal street market or online sales. The e-cigarette is also used for illicit drug delivery. THC and cannabinoid (CBD) oils are popular drugs. Other liquid components are synthetic cathiones, benzoylmethylecgonine (cocaine), gamma-hydroxybutyric acid (GHB), heroin, fentanyl, 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxymethamphetamine (MDMA) and methamphetamine.1
E-cigarettes for stopping or reducing tobacco smoking. E-cigarettes have mainly been promoted as a way of stopping or reducing tobacco smoking in smoking adults, especially in the early years after its first appearance in 2003. In a recent randomized trial of e-cigarettes versus nicotine-replacement therapy, the e-cigarette was shown to be more effective compared to nicotine-replacement therapy for smoking cessation, when both strategies were accompanied by behavioral support.2 Amongst young adults, the most important reason for e-cigarette use is experimentation, followed by stopping smoking.3
Presentation of VAPI. Although vaping has been promoted commercially as a safer alternative to traditional tobacco cigarettes, it has been shown to be associated with a large spectrum of lung injury. Symptoms may be limited to one organ such as the lung or cardiovascular system or combined respiratory, gastrointestinal and systemic. Dyspnea can be severe and rapidly progressive, leading to severe respiratory failure requiring intubation and/or extracorporeal membrane oxygenation ECMO and in some cases even resulting in a fatal outcome in previously healthy adolescents and adults. The Centers for Disease Control CDC has recently reported an epidemic of a spectrum of pulmonary diseases, associated with the use of e-cigarettes. Until December 2019 2409 cases of vaping associated pulmonary illness VAPI have been reported in the United States, also known as E-cigarette Vaping Associated Lung Injury EVALI, of which 52 2% had a fatal outcome.4
Aim of the review. This review aims to report what is currently known about vaping-associated pulmonary illness and includes the first reported case of VAPI in Switzerland.
MethodsWe searched original papers, observational studies, case reports, meta-analyses published between 2000 and end of 2019 in English in the PubMed database using the keywords: e-cigarette, "vaping-associated pulmonary illness", VAPI, EVALI, vaping AND “lung injury”. We also used data of the Centers of Disease Control (CDC) website and searched the reference lists of the retrieved articles.
ResultsFrom an initial search of PubMed, 62 potential articles were identified, and another 9 studies were identified from the bibliographies of retrieved articles. In this search we found 7 case series, 16 case reports, and 4 review articles. No randomized controlled studies were found. Available evidence on presenting features, imaging and pathology results as well as treatment strategies, are summarized in the descriptions below and in the respective tables (Tables 1–3).
Main references limited to original papers and case reports (excluding reviews, meta-analyses or commentaries).
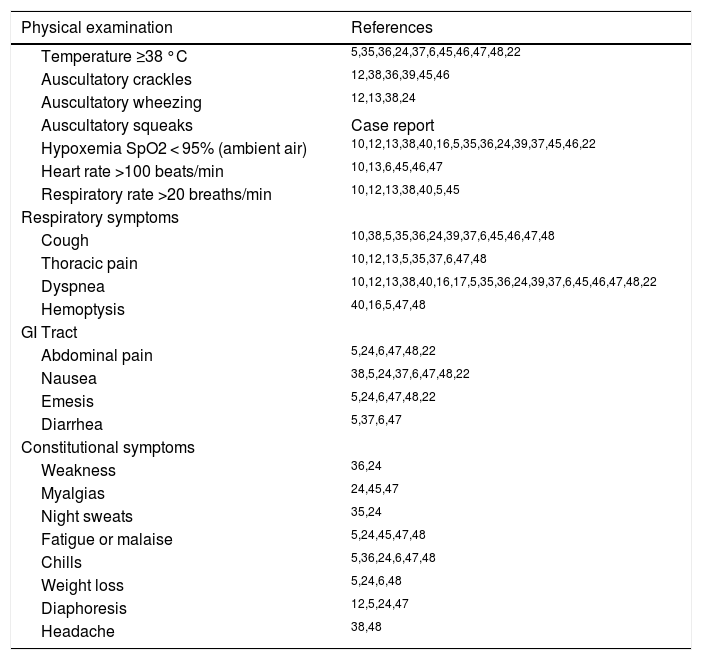
| Author, ref. and date | Study | No. of patients (median) age, sex | Type of e-cigarette | Specific treatments if present | Main outcomes | Hospi-talization (days) |
|---|---|---|---|---|---|---|
| Lozier4312/2019 | Case Series | N = 2.29124 yrs.67% male | most THC-containing products | N/A | 48 (2%) deaths of whom 54% male, median age 52 yrs. | Unknown |
| Sakla4410/2019 | Case Report | N = 125 yrs.Female | unknown | Azithromycin, ceftriaxone, levofloxacin, intubation, 3 weeks VV ECMO | ARDS, survived | >21 |
| Casanova4511/2019 | Case Report | N = 131 yrs.Female | Nicotine salts | Ceftriaxone, azithromycin, methylprednisolone 40 mg daily, increased to 80 mg daily, after 12 days oral prednisolone tapering dose | Bilateral pneumonia with pleural effusion | 12 |
| Landman4611/2019 | Case Report | N = 117 yrs.Male | Flavored e-liquids and THC | Mechanical ventilation, VV ECMO, high-dose steroids | Life-threatening bronchiolitisRemaining limited exercise capacity and fixed airflow obstruction with gas trapping | 47 |
| Kalininskiy4711/2019 | Case Series | N = 1227 yrs. (21-35)(21–35), 58% male, 42% female | 92% THC58% nicotine8% cannabidiol | 67% ICU admissionAntibiotics (92%)Steroids (67%)High flow 50%Mechanical ventilation 8%BiPAP 8%Nasal cannula 33% | Bilateral GGO (100%)Pleural effusions (9%)Fibrotic features (18%)Mediastinal lymphadenopathy (27%)No deaths50% resolution of previous chest CT findings and normal spirometry | 7 (7–8) |
| Blagev4811/2019 | Case Series | N = 6080% male, 20% female27 years (22-6) | 67% e-nicotine 78% THC, 48% both e-nicotine and THC, 5% e-cannabidiol oil | 90% antibiotics95% steroids88% supplemental oxygen47% high flow nasal cannula28% NIPPV17% mechanical ventilation55% ICU admission | Pneumothorax and pneumomediastinum, SIRS, ARDS, chemical pneumonitis, hypersensitivity pneumonitis, inhalational injury, lung abscess, infected pneumatocele.3% died (vaping considered a contributing factor but not the cause of death)10% readmission rate to ICU or hospital < 2 weeks (of whom 50% relapsed with vaping)residual abnormalities on chest radiographs (67%) and lung function (67%) 29% discharged on supplemental oxygen | 5 (3–8) |
| Davidson2209/2019 | Case Series | N = 518-35 yrs.(sex unknown) | THC (5), nicotine (3) | I.v. methylprednisolone (120−500 mg daily) | Lipoid PneumoniaNo deathsN = 3 ICU, N = 1 mechanical ventilation | Unknown |
| Flower1704/2017 | Case Report | N = 133 yrs.Male | “Tsunami White Spirits vaporizer E-Liquid E-Juice” | Quit vaping | RB-ILD, resolution after cessation of vaping | N/A |
| Sommereld1006/2018 | Case Report | N = 1Age 18 yrs.Female | Unknown | PICU admissionBroad-spectrum antibiotics40 mg methylprednisolone twice dailyMechanical ventilation for 5 daysVasopressor support with norepinephrine | Hypersensitivity pneumonitis and ARDS | Unknown |
| Moore1209/2015 | Case Report | N = 1Age 43 yrs.Male | Unknown | 2L nasal oxygenAntibiotics (not specified)Nebulized albuterol-ipratropium | Bilateral pneumonia and pleural effusions | 2 |
| Khan1312/2018 | Case Report | N = 1Age 40 yrs.Female | Unknown | Steroids | Organizing pneumonia | Unknown |
| Agustin1609/2019 | Case Report | N = 1Age 33 yrs.Male | Unknown | Steroids | Diffuse alveolar hemorrhage Symptoms improved with complete resolution of alveolar hemorrhage on chest CT scan after 2 weeks | Unknown |
| Khanijo5012/2019 | Case Series | N = 24Age unknown | THC | Unknown | Unknown | Unknown |
| Sharma4910/2019 | Case Report | N = 1Age 35 yrs.Male | “Heavy Hitters – Cold filtering cartridge”, mixture of 89% active cannabinoids, 86% THC with “distilled oil and terpenes” | Methylprednisolone 40 mg twice daily for 5 days followed by oral prednisolone Also, VATS with bleb removal and right-sided parietal pleurectomy | Chemical pneumonitis and pneumothoraxSurvived | Unknown |
| Buus5110/2019 | Case Report | N = 1Age 23 yrs.Male | marijuana | Prednisone 60 mg in tapering dose, and doxycycline | Diffuse tree-in-bud and nodular infiltrationSurvived | Unknown |
| Qarajeh5211/2019 | Case Report | N = 1Age 47 yrs.Male | THC | MethylprednisoloneIv furosemideCeftriaxone, azithromycin, vancomycin, piperacillin-tazobactam, levofloxacin | Multifocal GGO and nodular opacities with interlobar septal thickening, small pleural effusionSurvived | Unknown |
| Conuel5311/2019 | Case Series | N = 5Age 36 yrs.(23–55)80% male | CBD oilsTHC oilsMarijuana | Methylprednisolone and oral corticosteroids | Bilateral GGO with subpleural sparingAll survived | 80% hospital/ICU admission (3–7 days)20% supportive care at home |
| Abeles5411/2019 | Case Report | N = 1Age 18 yrs.Male | THC oils | AzithromycinLevofloxacinBiPAP | Bilateral GGOSurvivedAfter admission slightly reduced diffusion capacity (DLCO/VA 61%) | 25 d |
| Ocampo-Gonzalez5511/2019 | Case Report | N = 1Age 20 yrs.Male | THC oils, nicotine and marijuana | Steroids | survived | Unknown |
| Triantafyllou5612/2019 | Case Series | N = 6Age unknown100% male | Cannabis and nicotine | Antimicrobial therapy and corticosteroids | Multilobar GGO with subpleural sparing | 8d (median), ICU 2d (median) |
| He4003/2017 | Case Report | N = 1Age 54 yrs.Male | Cannabis | Antimicrobial therapy, high flow oxygen therapy | Focal airspace opacities and centrilobular nodular pattern (tree-in-bloom) | 5d |
Clinical presentations.
| Physical examination | References |
|---|---|
| Temperature ≥38 °C | 5,35,36,24,37,6,45,46,47,48,22 |
| Auscultatory crackles | 12,38,36,39,45,46 |
| Auscultatory wheezing | 12,13,38,24 |
| Auscultatory squeaks | Case report |
| Hypoxemia SpO2 < 95% (ambient air) | 10,12,13,38,40,16,5,35,36,24,39,37,45,46,22 |
| Heart rate >100 beats/min | 10,13,6,45,46,47 |
| Respiratory rate >20 breaths/min | 10,12,13,38,40,5,45 |
| Respiratory symptoms | |
| Cough | 10,38,5,35,36,24,39,37,6,45,46,47,48 |
| Thoracic pain | 10,12,13,5,35,37,6,47,48 |
| Dyspnea | 10,12,13,38,40,16,17,5,35,36,24,39,37,6,45,46,47,48,22 |
| Hemoptysis | 40,16,5,47,48 |
| GI Tract | |
| Abdominal pain | 5,24,6,47,48,22 |
| Nausea | 38,5,24,37,6,47,48,22 |
| Emesis | 5,24,6,47,48,22 |
| Diarrhea | 5,37,6,47 |
| Constitutional symptoms | |
| Weakness | 36,24 |
| Myalgias | 24,45,47 |
| Night sweats | 35,24 |
| Fatigue or malaise | 5,24,45,47,48 |
| Chills | 5,36,24,6,47,48 |
| Weight loss | 5,24,6,48 |
| Diaphoresis | 12,5,24,47 |
| Headache | 38,48 |
Radiological presentations.
| Hypersensitivity pneumonitis (HP)10 |
| Acute eosinophilic pneumonia (AEP)41,42 |
| Pneumonia with pleural effusions12 |
| Organizing pneumonia (OP)13 |
| Acute lung injury (ALI) and Acute Respiratory Distress Syndrome (ARDS)38,40,5,15 |
| Diffuse alveolar hemorrhage (DAH)16 |
| Respiratory bronchiolitis-associated pneumonitis (RB-ILD)17Giant-cell interstitial pneumonitis (GIP)14Acute lipoid pneumonia (LP)39,37Bronchiolitis (our case report),46 |
VAPI (and EVALI) is a diagnosis of exclusion. There are no specific clinical, laboratory, radiological or pathological markers of disease. There are case definitions, which are likely to evolve, as more information becomes known about the etiology and pathogenetical mechanisms involved. It is a potentially fatal complication of vaping and it can mimic or be associated with respiratory infections, and present with a great variety of symptoms (Table 1, Table 2).
For surveillance purposes, not for clinical diagnosis, the CDC described case definitions for "probable cases" and "confirmed cases".5 A “probable case” includes “using an e-cigarette (vaping) or dabbing in 90 days before symptom onset; and pulmonary infiltrate, such as opacities on plain film chest radiograph or ground-glass opacities on chest CT; and infection identified by means of culture or PCR, but the clinical team caring for the patient believes that this is not the sole cause of the underlying respiratory disease process; or as the minimum criteria, to rule out pulmonary infection not met (testing not performed) and clinical team caring for the patient believes that this is not the sole cause of the underlying respiratory disease process“.5
The case definition of “confirmed case” includes the same definition as above, but there should be “absence of pulmonary infection on initial workup: the minimum criteria include negative respiratory viral panel and influenza PCR or rapid test if local epidemiology supports testing. All other clinically indicated testing for respiratory infectious disease (e.g., urine antigen testing for Streptococcus pneumoniae and legionella, sputum culture if productive cough, bronchoalveolar lavage culture if done, blood culture, and presence of HIV-related opportunistic respiratory infections if appropriate) must be negative”.5
In both “confirmed” and “probable” cases, there should be no evidence in medical record of alternative plausible diagnoses such as cardiac, rheumatologic or neoplastic process (Table 4).
Pathology features.
| Cytology: bronchoalveolar lavage (BAL) | References |
|---|---|
| Lipid-laden macrophages | 10,22,24,28,37,39,45,53,54,55 |
| Foamy macrophages | 28 |
| Neutrophil predominant | 10,16,24,28,53 |
| Eosinophil predominant | 42 |
| Hemorrhagic | 16,40 |
| Non-diagnostic | 17,35,40,46,48,49 |
| Histology: Transbronchial biopsy (TBB) | |
| Acute alveolitis with intra-alveolar fibrosis | 15 |
| Diffuse alveolar damage (DAD) | 28 |
| Organizing pneumonia | 28,40 |
| Non-diagnostic | 17,46 |
| Histology: surgical biopsy | |
| Organizing pneumonia | 13,28 |
| Respiratory bronchiolitis-associated pneumonitis (RB-ILD) | 17 |
| Desquamative interstitial pneumonitis (DIP) | 17 |
| Diffuse alveolar damage (DAD) | 24 |
| Lipoid pneumonia | 35 |
According to data of the CDC, 95% (323/339) of patients diagnosed with VAPI initially experienced respiratory symptoms (e.g., cough, chest pain, or shortness of breath), and 77% (262/339) had gastrointestinal symptoms (e.g., abdominal pain, nausea, vomiting, or diarrhea).6 As documented in the CDC data, gastrointestinal symptoms preceded respiratory symptoms in some patients.6 Respiratory or gastrointestinal symptoms were accompanied by constitutional symptoms such as fever, chills, and weight loss in 85% (289/339) of patients to date.6 Of these patients, 47% were admitted to the ICU, and 22% needed intubation and mechanical ventilation.6 The clinical presentations are described in Table 2. On the other hand, there has been some concern about the association between the use of e-cigarettes and the reporting of a diagnosis of COPD in adults.7 Perez et al. found a significant association between using e-cigarettes every day or only sometimes and the reported diagnosis of COPD, even after adjusting for the use of combustible tobacco products and other risk factors associated with this condition. The subgroup analysis showed an association between the use of e-cigarettes and reporting COPD among subjects 35 years and older, 45 years and older and 55 years and older. There were also increased odds of reporting a COPD diagnosis for a subgroup of respondents who only used e-cigarettes and no combustible cigarettes.7 However, some results relating to the COPD development do not confirm this observation: One small 3.5-year prospective observational study, with nine daily e-cigarette users who had never smoked combustible cigarettes and a reference group of twelve smokers, did not show significant changes in blood pressure, heart rate, body weight, lung function, respiratory symptoms, exhaled breath nitric oxide, exhaled carbon monoxide nor high-resolution computed tomography findings in the lungs for both groups studied.8 The length of the observation period is certainly important when studying the effects of irreversible airway obstruction.
Radiological presentationsThe radiological patterns (Table 3) may depend on the frequency, dose and chemical characteristics of the inhaled substances, but also on the vaping device used.9 Generally, chest CT-scans can demonstrate bilateral ground-glass opacities (GGO), with sparing of the lung periphery, and centrilobular ground-glass nodules. Also, a crazy-paving pattern can be seen. The following interstitial pneumonias have been described in VAPI cases;
- a)
Hypersensitivity pneumonitis has been described after vaping, in which the chest CT scan showed dependent opacities in both lung bases, with superimposed smooth interlobular septal thickening, and pleural effusions.10
- b)
Acute eosinophilic pneumonia has been shown to present with ground-glass opacity and pleural effusion in 133 (97%) patients and 121 (88%) patients, respectively.11 Interlobular septal thickening and centrilobular nodules were present in 93 (68%) and 71 (52%) patients, respectively. Other findings were air-space consolidation in 51 (37%) patients and thickening of bronchovascular bundles in 24 (18%) patients. Interestingly, air-space consolidation was more frequently observed in patients with respiratory failure, and thus may be a prognostic marker of severity of the condition.
- c)
Pneumonia with pleural effusions has been described in a patient who had been smoking e-cigarettes for 3 days and had taken hundreds of puffs each day, using the electronic cigarette all day long without stopping until bedtime.12
- d)
Organizing pneumonia has been described after using e-cigarettes for 1 month, leading to respiratory failure with intubation and mechanical ventilation in a 40-year-old female.13 The most typical findings are bilateral patchy ground-glass opacities, consolidation, or both in a peripheral or perilobular distribution.14
- e)
Acute lung injury and Acute Respiratory Distress Syndrome (ARDS) have also been associated with vaping and VAPI.15
- f)
Diffuse alveolar hemorrhage (DAH) has mainly been associated with cocaine and cannabis use unrelated to vaping practices. One case report has related DAH to vaping, in a thirty-three-year-old male presenting to the emergency department with worsening dyspnea and hemoptysis.16 He admitted vaping for the past 2 months with overtly increased exposure time and had experimented new flavors. This male had no previous history of recreational drug use. The diagnosis of DAH was confirmed by bronchoscopy with BAL, ruling out other causes such as infection and vasculitis, and a right-sided wedge resection lung biopsy revealed bland pulmonary hemorrhage with no evidence of capillaritis or diffuse alveolar damage. Chest CT scan showed diffuse GGO and bilateral patchy consolidations, resulting in severe hypoxia requiring noninvasive ventilation. After treatment with corticosteroids the symptoms improved with complete resolution of alveolar hemorrhage on a chest CT scan two weeks after admission.
- g)
Respiratory bronchiolitis-associated pneumonitis (RB-ILD) was diagnosed in a 33-year-old male after 3 months of vaping while continuing to smoke 10 traditional cigarettes per day.17 This patient had a 10 PY smoking history. Chest CT showed multiple new poorly- defined pulmonary nodules with fluffy parenchyma opacification along the terminal bronchovascular units. Video-assisted thoracoscopy with lung biopsy of the right upper and middle lobes were performed, with microscopical confirmation of the radiological findings of RB-ILD. This interstitial lung disease was clearly linked to e-cigarette use, since the patient had had multiple CT scans previously due to prior treatment of a mixed germ cell tumor. It had also been documented that during treatment with bleomycin and continued smoking of traditional cigarettes, the CT scans had never shown evidence for RB-ILD in any of his previous chest CT scans.
- h)
Giant-cell interstitial pneumonia is a pneumoconiosis from exposure to hard metal.18 This rare diagnosis was made on the basis of findings in a surgical biopsy of the lung. The findings in this patient were attributed to hard metal (cobalt) contamination in her vape pen. The biopsy showed fibrosis characterized by peripheral reticulation, GGO and mild traction bronchiectasis. The patient’s symptoms improved after cessation of vaping.19 On the chest CT scan it is presented as GGO, architectural distortion and linear opacities in a peribronchiolar distribution.20
- i)
Acute lipoid pneumonia (ALP) has also been studied in pediatric patients with pneumonia caused by aspiration of oil-based substances. The predominant findings in chest CT scans were areas of consolidation. There were no case fatalities, and in the follow-up between 2 weeks to 6 months, all patients with clinical symptoms experienced remission. The CT scans of most of the cases had normalized by 1–3 months after the cessation of exposure with the exception for two patients who only showed complete improvement 6 months after treatment.21 In e-cigarettes the inhaled aerosolized oils can be deposited in the distal airways and alveoli, leading to an acute lipoid pneumonia by provoking a local inflammatory response that impairs vital gas exchange.22 Although not present in all cases, macroscopic fat attenuation within the consolidations (<-30 HU) are considered diagnostic for ALP.14 Typically, CT scans show ALP findings in the dependent lung, including GGO, consolidation, crazy-paving, or a combination of these.14
- j)
Bronchiolitis, with signs of air-trapping, inspiratory squeaks on auscultation are described in our case report (see below).
In general, the e-cigarette aerosol particles contain droplets in the 200 µm size range.23 Although only ultrafine particles, defined as particles smaller than 0.1 µm, have the ability to reach the blood circulation by passing through the lung tissue, the e-cigarette aerosol particles can at least reach the respiratory bronchioles which can lead to various outcomes.
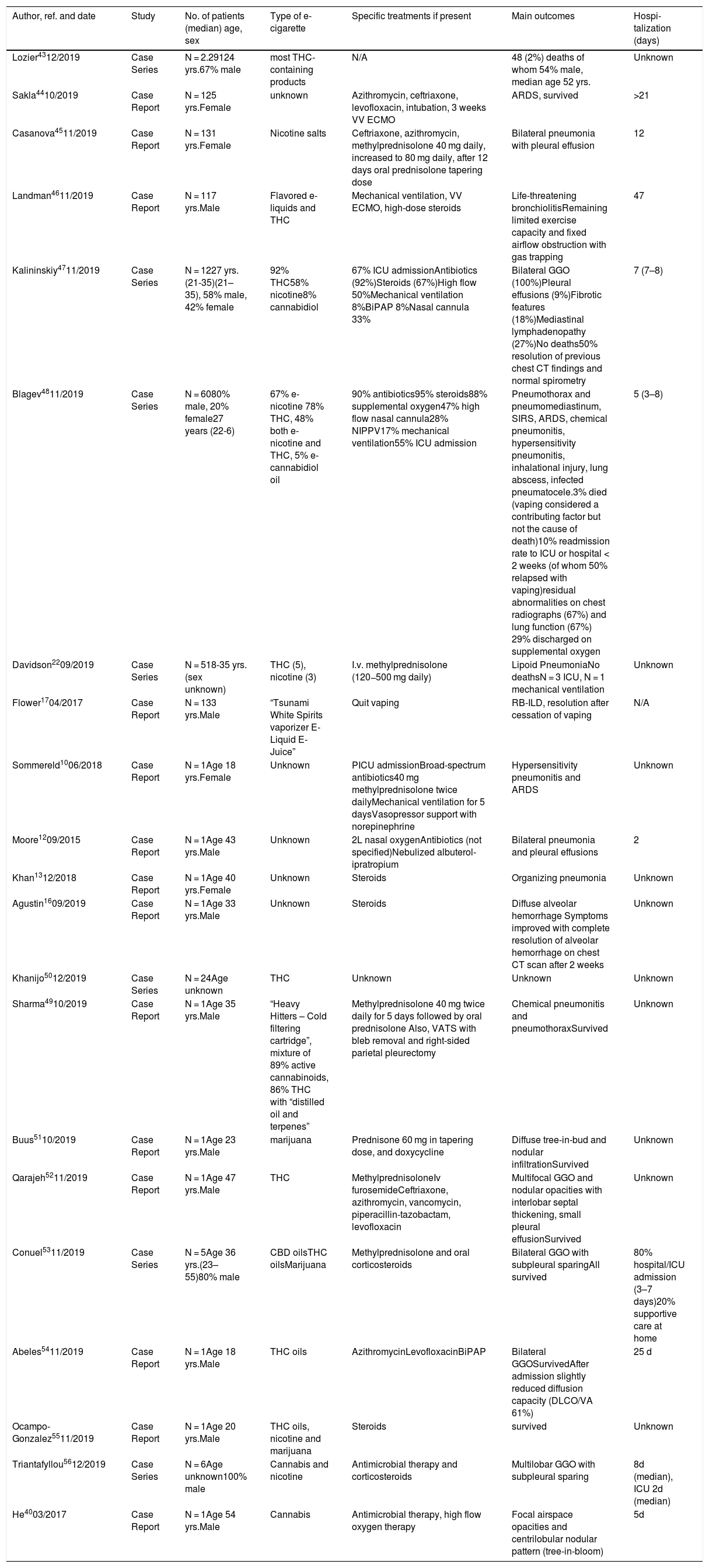
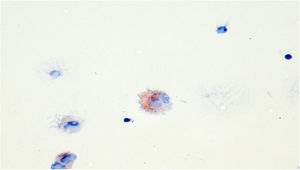
Routes of aerosol particlesOne possible route is removal by mucociliary clearance and phagocytosis by macrophages. In BAL samples macrophages have been shown to be lipid-laden, seen with Oil Red O staining (Fig. 3).10 The pathophysiological significance of these lipid-laden macrophages and their relation to VAPI are not known, but they may be a useful marker of this disease.24
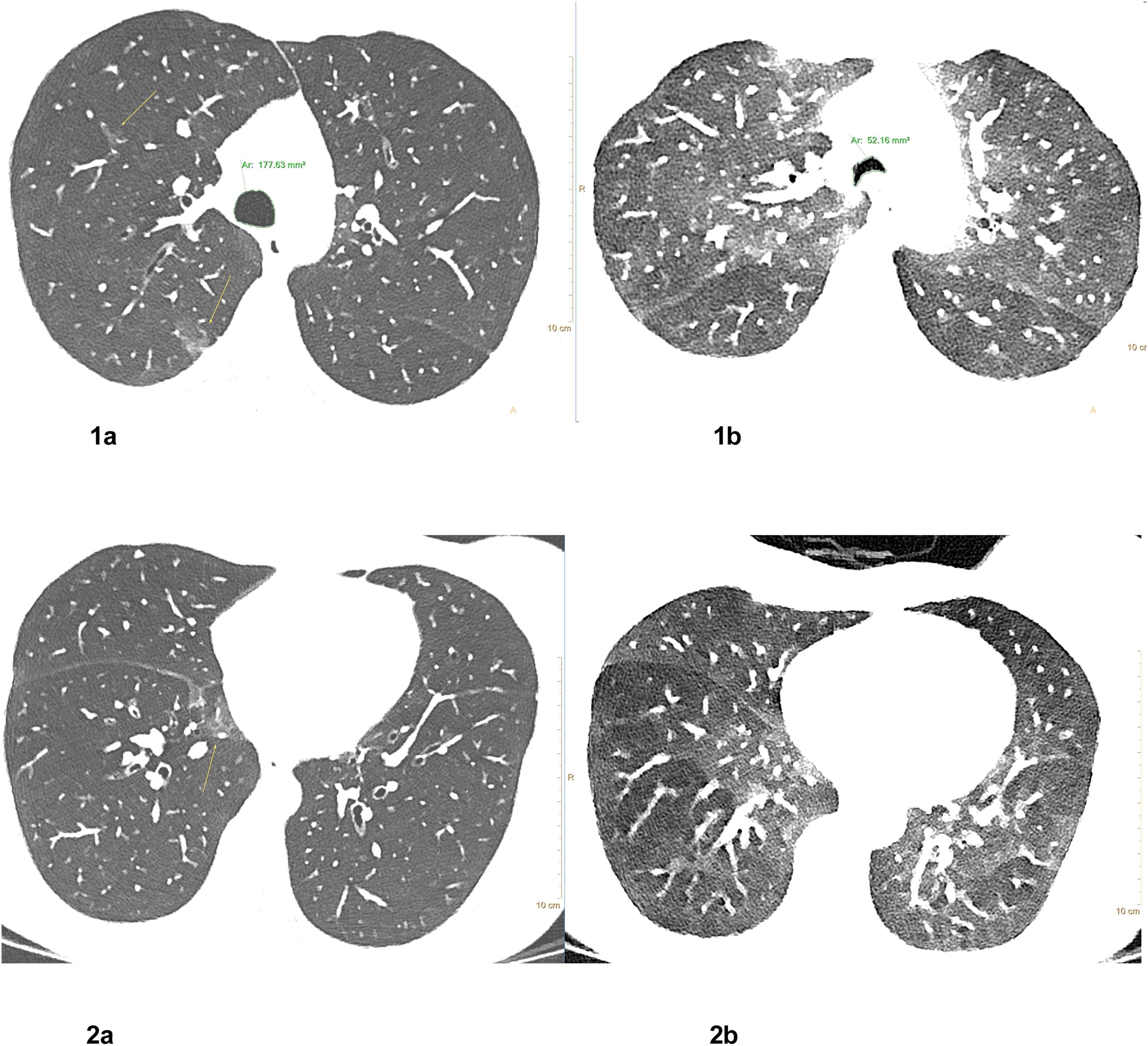
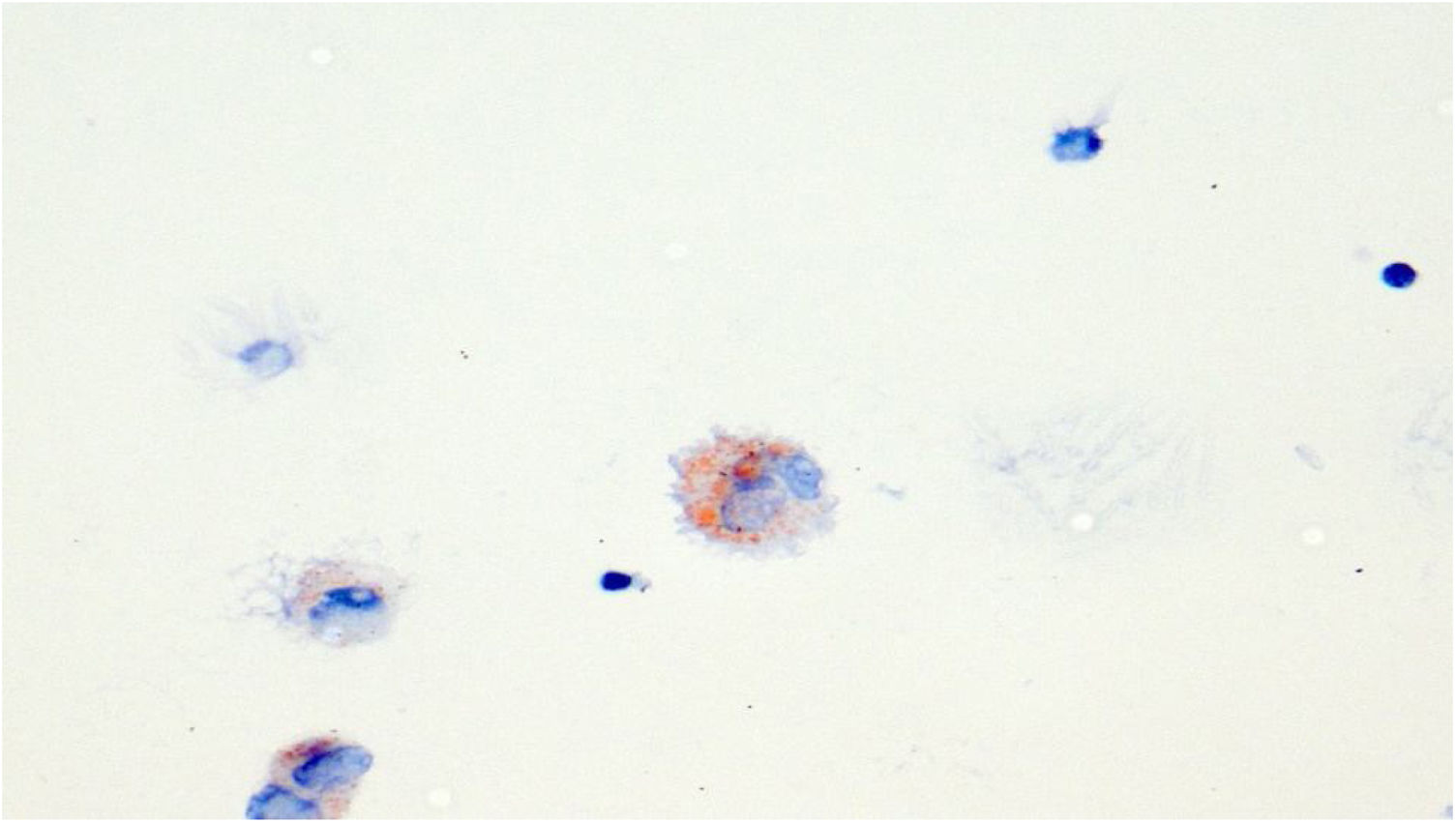
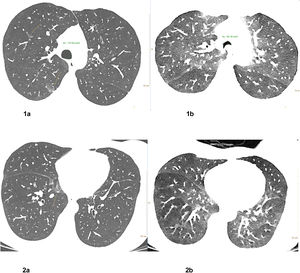
Lipid laden macrophages, Oil Red O staining. Chest CT 1 week after treatment onset: Corresponding axial CT images in inspiration (left row, Fig. 1a and 2a) and forced expiration (right row Fig. 1b and 2b) at 2 levels show small residual pulmonary ground-glass opacities (yellow arrows) in the medio basal right upper lobe and posterior right upper lobe, bronchial wall thickening, significant dynamic airways collapse (measurements of tracheal caliber, green) and mosaic attenuation with hypodense air trapping (images on the left).
Alternatively, these particles remain in the lung tissue or reach the regional lymph nodes. The depositing and clearance of these particles occurs in an inhomogeneous manner.25
Pathophysiological responsesAs yet, there is little information on the interaction of these particles with the pulmonary epithelium, regional lymph nodes, phagocytes or cytokines.
- a)
Cytokine responses and oxidative toxicity
One study showed measurable oxidative and inflammatory responses in lung cells, by exposure to vaporizing liquids, which caused in the human airway epithelial cells in vitro increased secretion of proinflammatory cytokines, such as IL-6 and IL-8, and lung fibroblasts exhibited stress and morphological changes.26 Moreover, in response to a specific flavored liquid (cinnamon), the airway epithelium secreted increased IL-8, and cells were shown to be susceptible to loss of cell viability.26 In mice, exposure of aerosols from e-cigarettes diminished lung glutathione levels, which are critical in maintaining cellular redox balance.26 The oxidative toxicity and inflammatory response, due to radicals and alteration in glutathione levels, probably have the same magnitude as in conventional tobacco cigarettes. The conventional cigarettes and e-cigarettes have approximately 1015 free radicals in a puff, as well as heavy metal nanoparticles. Although not proven yet, interaction of these particles with airway epithelium and phagocytes might lead to inflammation with clinically manifest flow limitation and chronic interstitial pulmonary fibrosis.
- b)
Lung injury
A recent study including lung biopsies (seven transbronchial, one surgical) showed acute lung injury including organizing pneumonia and/or diffuse alveolar damage.27 Common features were fibroblast plugs, hyaline membranes, fibrinous exudates, type 2 pneumocyte hyperplasia and interstitial organization, with macrophages present within the airspaces in all cases.27 Another study, reviewing the pathology of lung biopsies from 17 patients with confirmed or probable VAPI, showed histopathological patterns of acute lung injury, including acute fibrinous pneumonitis, diffuse alveolar damage, or organizing pneumonia, usually bronchiolocentric and accompanied by bronchiolitis.28 There were foamy macrophages and pneumocyte vacuolization in all cases.
- c)
Possible causative agents
Although no definitive conclusions can be drawn yet, nicotine products, tetrahydrocannabinol (THC), cannabidiol (CBD) and vitamin E are among the most suspected ingredients with a strong link to the pathogenesis of VAPI. However, a recent animal study showed that propylene glycol, as "carrier fluid" in e-cigarettes, induced rapid lung damage in just 3 days of e-cigarette use.29 In this study, mice were exposed to e-cigarette aerosols for two hours per day on three consecutive days. Comparable lung damage could be seen in mice inhaling e-cigarette aerosols with nicotine, and e-cigarette aerosols with only propylene glycol, showing that propylene glycol alone can lead to acute lung damage in mice. Female mice showed stronger inflammatory responses than males. Another study in mice has implicated nicotine as the cause for emphysema development.30 This to date has not yet been reproduced. The rapid lung damage was also described in a case report of a former smoker, who started excessive vaping 3 days before presenting to the emergency department and developed a bilateral pneumonia with pleural effusions.12 Recently, vitamin E has been found in BAL fluid samples (or samples of fluid collected from the lungs) from 29 patients with VAPI, while THC was found in 82% of the samples, and nicotine in 62%.31 Interestingly, also two flavorants, diacetyl and 2,3-pentanediol, could interfere with cilia and cytoskeletal processes in normal human bronchial epithelial cells, by interaction with gene expression pathways in cilia and cytoskeleton.32 The pathophysiology of VAPI remains largely unknown, but recent publications have helped to unravel some of the characteristic findings in these patients and vitamin E acetate seems to be one of the main culprit substances.
DiagnosisThe diagnosis of VAPI is a diagnosis of exclusion. Other diagnoses need to be excluded first.
- a)
History and physical examination
A detailed history is important and should include respiratory, gastrointestinal and systemic symptoms, smoking history, the vaping product (device), liquids and self-mixed liquids, especially asking for THC oils and possibly vitamin E containing products. On physical examination, beside vital signs including pulse oximetry, there can be signs of bronchial obstruction (wheezing) and bronchiolitis (end-inspiratory squeaks).
- b)
Laboratory and microbiological investigations
Laboratory investigations can be used to exclude other diseases and may show signs of infection. The routine investigation should include C-reactive protein and procalcitonin. In most patients generally there is a leukocytosis with predominant neutrophilia without eosinophilia, and an elevated C-reactive protein. Blood cultures, sputum Gram staining and sputum culture, arterial blood gas measurement should be considered. A urine drug screen, including testing for tetrahydrocannabinol (THC), as well as urine for Legionella and Pneumococcus antigen should be part of the routine examination if VAPI is suspected. A respiratory multiplex PCR (mPCR) to test for viral infections (depending on the season) including Influenza virus, may be considered for bronchoalveolar lavage fluid or nasopharyngeal swab or brush samples.
- c)
Radiological imaging
Radiologic imaging is mandatory, and at least one chest X-ray should be performed as an initial investigation. The chest X-ray can show bilateral infiltrates, sometimes additionally a pleural effusion. Interestingly, a case report of secondary spontaneous pneumothorax induced by vaping has been published49 and in the study of Blagev et al. there was pneumothorax and pneumomediastinum in 11 (18%) of 60 patients with VAPI.48 Since interstitial patterns can be very subtle or absent on chest X-ray examinations, a chest CT scan will be performed in most patients with progressive respiratory symptoms, especially when X-ray findings are discrete with a discrepancy to the severe clinical condition of the patient. The chest CT scan is useful to help choose the site of sampling during the bronchoscopy with bronchoalveolar lavage (BAL) or transbronchial biopsy. The chest CT scan also helps rule out other pulmonary diseases, such as infection or malignancy. If bronchiolitis is suspected, additional expiratory scans should be requested to document air-trapping.
- d)
Bronchoscopy
Bronchoscopy is useful to obtain pulmonary samples. However, depending on the condition of the patient, it may not be immediately possible and might lead to a further deterioration and intubation. Once intubated or on ECMO, bronchoscopy is advisable, since sampling results may help exclude or diagnose alternative diagnoses and thus influence treatment options. Bronchoscopy is likely to influence management of patients with atypical radiological findings, such as cavitation or large nodules, recent exposure to unusual pathogens, immunocompromised patients or in those already intubated. The decision when to perform a bronchoscopy will also depend on the clinical stability of the patient. In the series of Layden et al. only 45% of the patients underwent a bronchoscopy.5 In addition to the standard BAL fluid analysis the Oil Red O or Sudan black staining should be considered in order to detect lipid-laden macrophages. The relevance of this finding is currently not known but it has been described in a number of VAPI cases.24 In the case of DAH a Prussion blue iron staining should be considered.
- e)
Clinical monitoring
Hospitalized patients need frequent monitoring, as lung injury in VAPI can lead to rapid deterioration within 24−48 h. This is in line with the CDC recommendation, that patients, who are not admitted to the hospital, should be scheduled for follow-up within 24−48 h. In hospitalized patients, consulting a critical care specialist is advised, because the risk of rapid deterioration leading to admission to an intensive care unit (ICU) and subsequent need of intubation with mechanical ventilation is high. In the CDC report, there was a 47% ICU admission rate (159/342 patients) and a 22% mechanical ventilation rate (74/338 patients).6
TreatmentTreatment of VAPI has so far been largely empirical (Table 1). There are no published trials investigating the state-of-the-art treatment of VAPI. First of all, patients should immediately refrain from vaping. Secondly, patients should receive oxygen support as needed, by nasal cannula, an oxygen mask, high-flow nasal cannula, non-invasive ventilation, mechanical ventilation or even ECMO. Thirdly, high dose systemic corticosteroids (e.g. initially intravenous methylprednisolone, followed by 1 mg/kg prednisolone daily) should be considered (Table 1). As shown by the CDC data of 140 patients with corticosteroid treatment, there was clinical disease improvement in 82%.6 Early initiation of antimicrobial coverage (e.g. cephalosporines i.v. and macrolides p.o.) for community-acquired pneumonia should always be considered for patients with severe clinical symptoms, as VAPI can be associated with concurrent respiratory infections. If clinically and/or radiologically suspected, monotherapy with macrolides may be a useful treatment option in case of bronchiolitis. In patients with severe bronchial obstruction, ipratropium/salbutamol inhalations will be useful. Prognosis is relatively good, even in severe disease, although fatalities have been described (see above). The mean duration of hospitalization overall was 6.7 days; in the age group of ≥ 51 years it was 14.8 days.6
Case reportA 44-year-old female former cigarette smoker with a 3 pack-year smoking history, was referred to our hospital because of severe dyspnea (mMRC 3–4), coughing with yellowish phlegm, wheezing and dyspepsia, not responding to high doses of corticosteroids and a trial of amoxicilline-clavulanic acid. She quit smoking by using a nicotine-containing liquid and a 3rd generation vaporizer.
The initial symptoms started two months after using e-cigarettes with nicotine-free liquids. The GP had been treating her for several months with high dose inhaled fluticasone propionate/formoterol, montelukast and multiple oral steroid courses, interpreting her symptoms as newly diagnosed adult-onset asthma, with spirometry showing an obstructive ventilatory pattern of variable severity (FEV1 ranging from 52%–102% on various occasions). She was afebrile and normotensive. Furthermore, lung auscultation revealed bilateral upper and lower zone end-inspiratory squeaks, suggesting a bronchiolitis. Pulmonary function tests showed a severe obstructive ventilatory defect (FEV1 0.94L, 42%), FeNO-measurement was attempted but was not feasible due to severe dyspnea. Blood gas showed a respiratory alkalosis with metabolic compensation, with relevant hypoxemia (pO2 7.3 kPa). The patient, who refused hospitalization, was instructed to stop vaping immediately. A 7-day course of azithromycin 500 mg once daily and salbutamol/ipratropium with an electrical nebulizer was initiated. The laboratory findings showed no elevated infection parameters (C-reactive protein <1 mg/l, no leukocytosis). At this time the patient was already on high dose systemic corticosteroids. The electrolytes, liver- and renal function were within normal limits. CT imaging, which was performed 7 days after presentation in our clinic and a 7 days abstinence from e-cigarettes, treatment with azithromycin, high dose oral corticosteroids and one intravenous methylprednisolone dose, demonstrated air-trapping and residual ground-glass opacities. (Figs. 1 and 2). Bronchoscopy showed lipid-laden macrophages by Oil Red O staining and normal eosinophils, neutrophils and lymphocytes in the BAL (Fig. 3). Transbronchial biopsy demonstrated local chronic inflammation with thickening of the basal membrane and fibroelastosis.
The respiratory multiplex PCR (mPCR), able to detect multiple respiratory viruses in a single assay, revealed a human metapneumovirus (hMPV) in the BAL specimen. Bacterial examination showed normal flora in both BAL and bronchial washes. After cessation of vaping, the initiated therapy including continued corticosteroids and the addition of azithromycin and salbutamol/ipratropium nebulisations, led to fairly rapid improvement of symptoms within 10 days, and the lung function showed complete normalization at follow-up.
DiscussionThis paper provides an update on the current knowledge of VAPI and a case report of a probable case of VAPI in Switzerland. VAPI can present various degrees of severity and a variety of clinical and radiological patterns.
- a)
VAPI classification according to CDC, Naranjo ADR and CTCAE
Although not meant for clinical diagnosis, according to the case definition of the Centers for Disease Control and Prevention, the interstitial lung disease in our patient can be classified as a “probable case” of severe pulmonary disease associated with e-cigarette use.5 In this patient, the 4 criteria of a “probable case” were met. First of all, the patient was using an e-cigarette within 90 days before symptom onset. Secondly, the chest CT shows ground-glass opacities. Thirdly a pulmonary infection was not the sole cause of the underlying respiratory disease process. The patient did not respond to broad-spectrum antibiotics and had severe dyspnea, with a discrepancy between the severe dyspnea and the amount of the pulmonary ground-glass in the chest CT. The bronchoalveolar lavage culture was negative for bacteria and viruses except for detection of hMPV. There is no evidence in the medical record of alternative plausible diagnoses such as cardiac, rheumatologic or neoplastic processes.
In addition, on the Naranjo ADR Probability Scale, used for scoring the risk of adverse drug reactions (ranging from Score 0, "Doubtful" to Score 9 "Highly Probable"), the e-cigarette would be classified as Score 3 (Possible).33
The severity of the dyspnea would be classified as Grade 4 (severe) according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 of the U.S. Department of Health and Human Services.
- b)
Acute bronchiolitis in VAPI: potentially life-threatening
The diagnosis of VAPI remains challenging. Specific clinical, laboratory, radiological or bronchoscopic findings have not yet been identified, despite analysis of many aspects so far, and many symptoms are similar to those of other lung conditions. In this respect the clinically and radiologically detected signs of bronchiolitis in our patient might be of special interest. The e-cigarette aerosol particle distribution is not homogeneous. Especially in the respiratory bronchioles, concentrations typically are 25–100 times the concentration seen in the mainstem bronchus, and similarly, in one publication,34 high concentrations were found in the large airway carinas. Extremely high concentrations of e-cigarette aerosol might have provoked the clinical manifestations of bronchiolitis, which was the striking finding in our patient at presentation in our clinic. The patient had a productive cough, and the e-cigarette particles could have interacted with airway mucus secretion, further increasing local inflammatory responses resulting in airway obstruction. Bronchiolitis has also been an important histopathological pattern found in lung biopsies of patients with VAPI.28 Acute bronchiolitis in VAPI is very rare. There is recent case publication of a 17-year-old patient with a life-threatening bronchiolitis related to electronic cigarette use, which was treated with ECMO.46
- c)
Additional investigations in VAPI
As reported in literature, laboratory findings in patients with VAPI are non-specific. Many patients show elevated infection parameters, such as leukocytosis and elevated C-reactive protein or erythrocyte sedimentation rate. However, our patient had been pretreated with multiple courses of high doses of corticosteroids and also short courses of antibiotics which possibly explain the absence of systemic inflammatory signs. Another explanation could be the abnormal behavior of inflammatory markers in VAPI. High false positive rates of procalcitonin and C-reactive protein has been observed in VAPI, limiting the use as infection parameter. It has been shown to normalize relatively rapidly and has been suggested as a supplementary diagnostic tool in the evaluation of patients with VAPI.50
Radiological findings can show a wide variety of lung injury patterns as discussed above (Table 3).20 In the study of Layden et al., patients with VAPI showed an abnormal chest radiograph in 91%.5 In our patient, after cessation of e-cigarette use no specific radiological pattern could be diagnosed, with the exception of ground-glass opacification in the mediobasal right lower lobe and diffuse air-trapping.
Bronchoscopy with BAL and/or transbronchial biopsy, should be performed to rule out infection, if the condition of the patient allows these investigations. In our patient, bronchoscopic findings are in line with the literature, reporting a median neutrophil value of 65% (range, 10–91) and a total of 7 of the 14 cytology reports on BAL specimens noted lipid-laden macrophages with Oil Red O stain, as observed in our patient.5
Although there is a continuing effort in this area, the elucidation of the pathogenetic mechanisms is an unmet need and is key to guiding treatment decisions. In the meantime, healthcare providers should strongly discourage patients from using these heavily marketed devices and warn their patients of the dangers of VAPI. While there is limited evidence that e-cigarettes are an effective strategy to quit smoking, there are alternative evidence-based methods that are safe and effective.
In summary, we describe the clinical and radiological presentations of VAPI cases published to date with a summary of the likely pathogenetical mechanisms leading to this clinical syndrome. The current treatment strategies used so far to address those patients presenting with signs of respiratory failure are summarized. We report a case of probable VAPI, presenting with symptoms and imaging compatible with a bronchiolitis with a complete recovery, following cessation of vaping in combination with corticosteroid, azithromycin and symptomatic treatment measures to address the imminent respiratory failure and severe airway obstruction in a previously healthy woman.
Conclusion: the authors’ viewSome patients experience negative pulmonary and systemic adverse effects from using vaping devices. The definitive causative agent has yet to be determined. A number of possible culprit factors (including vitamin E acetate) have been suggested and empirical treatment strategies have emerged including cessation of vaping, corticosteroid, anti-infective and supportive measures.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Cynthia Ball for the English revision and editing of the manuscript.