An outbreak of Legionella pneumophila serogroup 1, with 403 cases was identified on the 7th November 2014 in Vila Franca de Xira, Portugal. Outbreak source was the wet cooling system of a local factory. Hospital Pulido Valente was one of the hospitals receiving patients with Legionnaires’ disease (LD).
MethodsWe describe the clinical findings and diagnostic methods used among the 43 confirmed or probable cases admitted to our department.
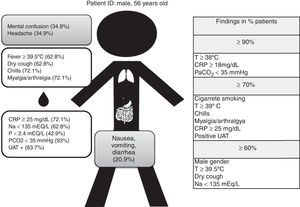
Results60.5% were male, mean age was 56.1±13.5 years and tobacco smoking was the most frequent risk factor (76.7%). All patients had fever, 62.8% ≥39.5°C, 72.1% had chills and myalgia/arthralgia and 62.8% had dry cough. Extra pulmonary symptoms were frequent: confusion and headache occurred in 34.9% and gastrointestinal symptoms in 20.9%.
High C-Reactive Protein (55.8% ≥30mg/dL) and hyponatremia (62.8%) were the laboratorial abnormalities most commonly found. Hypoxemia occurred in 55.8% and hypocapnia in 93%. Urinary Antigen Test (UAT) was positive in 83.7% of the cases.
ConclusionsAlthough not specific, a combination of risk factors, symptoms and laboratory findings can be highly suggestive of LD, even in an outbreak. This should prompt diagnosis confirmation. Routine use of UAT in less severe cases of community acquired pneumonia might contribute to earlier diagnosis.
Legionella species (spp.) is a relatively common agent of community and hospital acquired pneumonia.1 It was first identified as the pathogen responsible for a pneumonia outbreak during an American Legion Convention in July 1976 in Philadelphia. Hence its name, Legionnaires’ disease (LD).2 It occurs in outbreaks or sporadically.3,4
Bacteria of the genus Legionella include 56 species and more than 70 serogroups. The most common specie is Legionella pneumophila, serogroup 1 which accounts for 90% of cases of LD and was the agent responsible for the 1976 outbreak.5,6Legionella pneumophila serogroup 1 is further divided into multiple subtypes.
Although L. pneumophila serogroup 1 accounts for 90% of cases in Europe and America, this incidence could be lower in some countries, such as Australia and New Zealand, where Legionella longbeacheae accounts for 30% of cases.7
The incubation period is usually between 2 and 10 days.4,8 In about 10% of cases the incubation period can be longer, lasting more than 10 days and up to 19 days.9,10
Diagnosis is based on clinical suspicion together with laboratory confirmation. Legionella culture of a respiratory specimen and urinary antigen testing are the most widely used.4,8 Although the clinical manifestations are non-specific, a combination of risk factors, symptoms and laboratorial findings can be suggestive of LD.11–13 It usually presents as severe pneumonia with a particular pattern of multisystem dysfunction. As with other pneumonias caused by “atypical pathogens”, respiratory symptoms may not be the most prominent. Neurological and gastrointestinal involvement, myalgia and arthralgia, hepatic cytolysis, hyponatremia, hypophosphatemia and renal failure are frequently present.4,11–15
An outbreak of L. pneumophila serogroup 1 with 403 cases, 377 confirmed and 26 probable cases, was identified on the 7th November 2014 in Vila Franca de Xira, located 30 Km north of Lisbon, Portugal.16 The outbreak started on the 12th October, with peak incidence on the 6th November. Control of outbreak was achieved on the 21st November, with its end declared on the 2nd December (date of start of symptoms of the last case). Of the total of 403 cases, there were 14 reported deaths, leading to a 3.5% overall case fatality rate. Epidemiological investigation identified the most probable outbreak source as the wet cooling system of a local factory. These facilities were shut down on the 9th November.17 Interestingly it was during this outbreak that the very first probable person to person transmission of LD has been described.18
During the outbreak several hospitals were appointed to receive patients from Vila Franca de Xira, and Hospital Pulido Valente – Centro Hospitalar Lisboa Norte was one of them. The authors describe the clinical findings and diagnostic methods used among the 43 confirmed or probable cases of LD admitted in our trust.
Materials and methodsForty-three patients with confirmed or probable LD were admitted to the Chest Department at Hospital Pulido Valente between the 7th and 15th November 2014. Case definition was based on established criteria by the Legionnaires’ Disease Surveillance European Union (EU) which was adopted by the Portuguese Directorate General of Health (DGH) (Table 1).8,19
Outbreak case definition adopted by Portuguese Directorate-General of Health based on the Legionnaires’ Disease Surveillance European Union (EU) case definition criteria.
| Case definition | Probable case: Any person meeting the clinical and epidemiological criteria and at least one laboratory criterion for a probable case Confirmed case: Any person meeting the clinical and epidemiological criteria and at least one laboratory criterion for a confirmed case |
| Clinical criteria | Any person with pneumonia |
| Epidemiological criteria | Symptom onset occurring between 12/10/2014 and 02/12/2014 and history of living, working or visiting at risk areas7 during the period of 2 to 19 days before symptom onset. |
| Laboratory criteria for confirmed case | At least one of the three following laboratory findings: a) isolation of Legionella spp. from respiratory secretions or any normally sterile site; b) detection of Legionella pneumophila antigen in urine; c) significant rise in specific antibody level to Legionella pneumophila serogroup 1 in paired serum samples. |
| Laboratory criteria for probable case | At least one of the following four laboratory findings: a) detection of Legionella pneumophila antigen in respiratory secretions or lung tissue e.g. by DFA staining using monoclonal-antibody derived reagents; b) detection of Legionella spp. nucleic acid in respiratory secretions, lung tissue or any normally sterile site; c) significant rise in specific antibody level to Legionella pneumophila other than serogroup 1 or other Legionella spp. in paired serum samples; d) single high level of specific antibody to Legionella pneumophila serogroup 1 in serum. |
A questionnaire was applied to all patients transferred from the at risk areas with a clinical suspicion of LD. Data regarding demographics, risk factors, clinical presentation, laboratorial and radiological features and microbiological testing was collected and analyzed.
A smoker was defined as a person who, at the time of the survey, had smoked at least 100 cigarettes in his/her lifetime and currently smokes cigarettes every day or occasionally. An ex-smoker was a person who used to be a smoker but had not smoked at all for at least 6 months at the time of the survey.20
Statistical analysis was performed aimed at a descriptive analysis of the LD cases. More specifically for categorical variables absolute and relative frequencies were calculated, for numerical variables means, medians and standard deviations (sd) were used to characterize their distribution. All data analysis was performed using Microsoft Excel (2007).
ResultsOf the 43 patients, 38 (88.4%) were confirmed cases and 5 (11.6%) were probable cases. The total of 43 cases represents around 10.7% of the total 403 cases identified during the outbreak.
All patients received levofloxacin and in our series there were no deaths. Seven patients were admitted to the Intensive Care Unit (ICU) and 11 to the Intermediate Care Unit (ITCU). Invasive Mechanical Ventilation (IMV) was needed in two patients and Non Invasive Ventilation (NIV) in eight patients. All patients were admitted after the outbreak had been declared. The majority was first seen in the Emergency Room (ER) closest to the outbreak source (Hospital de Vila Franca de Xira).
Fever was present for a mean of 4.0 days before patients sought medical assistance. Every patient with suspected LD and epidemiological link was medicated with high dose levofloxacin (750mg) before being transferred to our hospital. Patients were maintained on monotherapy with high dose levofloxacin for a mean period of 8.9 days.
Demographic characteristics and host risk factorsDemographic characteristics and host risk factors are presented in Table 2. Twenty-six patients were male and 17 were female. Mean age was 56.1 and median age was 59 years old. The youngest and oldest patients were 22 and 85 years old, respectively. Around three quarters were of active age: 74.4% were within 35–65 years.
Demographics and host risk factors of the 43 confirmed/probable patients.
| Characteristics | Confirmed/probable cases N (%) |
|---|---|
| Gender | |
| Male | 26 (60.5) |
| Female | 17 (39.5) |
| Age (mean±SD) | 56.1±13.5 |
| Age median | 59 |
| Active smoker or ex-smoker | 33 (76.7) |
| Chronic respiratory disease | 9 (20.9) |
| COPD | 6 (14.0) |
| Asthma | 2 (4.7) |
| OSAS | 2 (4.7) |
| Diabetes mellitus | 7 (16.3) |
| Active cancer/Malignancy | 1 (2.3) |
| Chronic therapy with systemic steroids or other immunosuppressive treatment | 0 |
| ≥1 risk factor | 10 (23.3) |
SD: standard deviation; COPD: Chronic Obstructive Pulmonary Disease; OSAS: Obstructive Sleep Apnea Syndrome.
The main risk factors identified were tobacco smoking (active smokers or ex-smokers) accounting for 76.7% of patients, chronic respiratory disease (20.9%) and diabetes mellitus (16.3%). More than 1 risk factor was present in 23.3% of patients. One patient had gastric cancer and none were under steroids or other immunosuppressive treatment. HIV serology was performed in 35 patients (81.4%). HIV-2 serology was positive in one patient with a CD4+ lymphocyte cell count of 454cells/μL. Although not established as a risk factor, 56.8% had cardiovascular disease, mainly hypertension.
Clinical, laboratorial and radiological presentationClinical features, radiological and laboratory results are presented in Table 3. All patients had fever (T≥38°C), in 79.1% of patients ≥39°C, in 62.8% ≥39.5°C and maximum temperature was 40.5°C.
Clinical and laboratory features.
| Characteristics | Confirmed/probable cases N (%) |
|---|---|
| Clinical features (all items were collected separately) | |
| General symptoms and signs | |
| Fever T ≥38°C | 43 (100.0) |
| Fever T ≥39°C | 34 (79.1) |
| Fever T ≥39.5°C | 27 (62.8) |
| Chills | 31 (72.1) |
| Myalgia/arthralgia | 31 (72.1) |
| Lethargy/tiredness | 19 (44.2) |
| Respiratory symptoms | |
| Dry cough | 27 (62.8) |
| Productive cough | 7 (16.3) |
| Dyspnea | 17 (39.5) |
| Chest pain | 14 (32.6) |
| Neurological symptoms | |
| Headache | 15 (34.9) |
| Mental confusion | 15 (34.9) |
| Gastrointestinal symptoms | |
| Nausea | 9 (20.9) |
| Vomiting | 9 (20.9) |
| Diarrhea | 9 (20.9) |
| Laboratory results | |
| CRP ≥18mg/dL | 39 (90.7) |
| CRP ≥25mg/dL | 31 (72.1) |
| CRP ≥30mg/dL | 24 (55.8) |
| Leukocytosis (>12×109cells/L) | 21 (48.8) |
| Platelets <171×109cells/L | 11 (25.5) |
| Na <135mEq/L | 27 (62.8) |
| P <2.4mEq/L | 9 (42.9) performed in n=21 |
| AST >34U/L | 23 (54.8) performed in n=42 |
| Creatinine >1.3mg/dL | 22 (51.2) |
| CK >294U/L | 10 (28.6) performed in n=35 |
| Arterial Blood Gas resultsa | |
| PO2 <60mmHg | 13 (30.2) |
| PO2 <70mmHg | 24 (55.8) |
| PCO2 <45mmHg | 43(100.0) |
| PCO2 <35mmHg | 40 (93) |
Chills, myalgia and arthralgia were present in 72.1% of the patients. Dry cough was the most frequent respiratory symptom (62.8%) and productive cough was the least (16.3%). Both dyspnea and chest pain were present in less than 40% of the cases. Extrapulmonary symptoms were frequent, with 34.9% of patients presenting with confusion and/or headache. Gastrointestinal symptoms, such as nausea, vomiting and diarrhea were reported in 20.9% of the patients.
Fifty-six percent of the patients had C-Reactive Protein (CRP) above 30mg/dL (mean value of 33.8, minimum 14.3 and maximum 66.9mg/dL). Leukocytosis was found in 48.8% of the patients (mean value of 12210×109cells/L). Normal platelet count (150–450×109/L) was found in the majority of patients (n=36, 83.7%) and 22.5% had platelet count below 171×109/L. The most common laboratory abnormalities were: hyponatremia (Na <135mmol/L) in 62.8%, with Na <130 in 20.9%, hepatic cytolysis (AST >34U/L) in 54.8%, raised creatinine in 51.2%, and hypophosphatemia in 42.9% of the cases.
All patients had arterial blood gas samples drawn on room air (FiO2 21%) on admission. Hypoxemia (PO2 <70mmHg) was found in 55.8% of the patients and 93% had hypocapnia (PCO2 <35mmHg).
All patients had a chest X-ray on admission documenting evidence of pneumonia. X-ray was repeated within 72h, despite clinical evolution. Fourteen percent (n=6) had bilateral involvement at admission and half of these patients were treated in the intermediate/intensive Care Units. Twenty-three percent (n=10) had worsening radiological features within the first 72h of hospitalization and half of them were admitted to the ICU.
Microbiological testingAll patients had Urinary Antigen Testing for L. pneumophila serogroup 1 (Alere BinaxNOW®Legionella Urinary Antigen Card). This was positive in n=36 (83.7%). In the 36 confirmed cases by UAT, serology was not performed and respiratory secretions were collected in 15 patients, and culture was positive in 6. Among the 7 patients with negative UAT, there were two additional confirmed cases: one by isolation of Legionella spp. from respiratory secretions and one by analysis of significant serological titer elevation in paired samples. The 5 remaining cases were probable cases, with a single high level of specific antibody to L. pneumophila serogroup 1 in serum.
Blood cultures were performed in all patients and negative in all for any pathogen, specifically for Streptococcus pneumoniae.
Fig. 1 shows the standard demographic and clinical findings of LD in our studied population (our “typical” patient).
DiscussionTo date, the largest outbreak of LD happened in Murcia, Spain in 2001 with 449 confirmed cases. The Vila Franca de Xira outbreak, with 403 cases, was the second largest.16,17,21
The outbreak started on the 12th October 2014 but was only declared, 26 days later, on the 7th November.8 Our study was not able to identify the reason for the delay in declaring the outbreak, but we hypothesize that this could be related to the high population density of the area affected by the outbreak and the number of different health care facilities involved. Other reasons include the fact that there are no national recommendations in Portugal for requesting UAT in patients with Community Acquired Pneumonia. As for this, UAT is performed in severe cases, those admitted to ICU or during an outbreak, as recommended by the Portuguese Respiratory Society guidelines for the management of community–acquired pneumonia in immunocompetent adults.22
As the outbreak had already been declared when the patients were admitted to our hospital, UAT was performed on all of them, regardless of disease severity.
Although our population of patients amounted to 43 cases (10.7% of the total number of cases) we believe it is representative of the clinical manifestations of LD, which are in line with the literature. The distribution of gender and age reflect the demographic characteristics of the population exposed to contamination. This is mainly a working population in an industrial area, many of whom commute to work in the capital.
All series report a greater incidence in males11,14,23,24 although the age distribution in our series was younger with 74.4% of cases being between 35 and 65 years of age with a mean age of 56.1 years. The Murcia outbreak showed an older population with 70% of patients being older than 5021 and in the review by Sopena 52.2% where older than 60.3 In the analysis of CAPNETZ by von Baum et al., the mean age was 63.25
Our series showed that the most prevalent risk factor was smoking history (current or past) which was present in 76.7% of patients. Again this was in accordance with previous series.3,25–27 Chronic respiratory diseases, frequently quoted as a risk factor, 9.7% in the Sopena series3 and 36% in von Baum's,25 was identified in 20.9% of our population In our series, diabetes mellitus was found in 16.3% of patients. Similar values were also found in other studies.3,13,26 However, diabetes mellitus prevalence in the Portuguese population aged 20–79 years old was 13.1% in 2014.28 Also, in an outbreak of LD in 2000 in Melbourne, Australia, a case–control study did not find significant difference in risk factors between patients and the general population, with the exception of smoking.26
The low incidence of immunosuppressive therapy and/or active cancer as well as a high incidence of high blood pressure in the population reflect the demographic characteristics and is likely to have no other significance. It is unknown what the HIV prevalence was in the population exposed to LD outbreak, however the patient diagnosed with HIV-2 had a CD4 lymphocyte cell count ≥350cells/μL. Thus, in this patient HIV infection probably did not contribute significantly as a risk factor for LD, which remains in accordance to other published data.29
All patients had fever, ≥39°C in nearly 80% of patients. According to some authors this alone should raise the suspicion of LD.15 As expected, respiratory symptoms were not predominant, with the most common being a dry cough in two thirds of patients.15,30 Chills, myalgia and arthralgia were predominant in our series (72.1% of cases) and is documented in the literature,15 however the latter was more prevalent when compared with other studies that quoted a prevalence around 40%.15,30,31
Around one third of patients had neurological complaints (headache and/or confusion) and 20.9% had gastrointestinal symptoms, mainly diarrhea, nausea and vomit. These values are similar to values from other studies.15,23,30,32
Regarding laboratory findings our series confirms very high CRP (mean value: 33.8mg/dL) which is in line with other series.13,33,34 Other findings already described in the literature include hyponatraemia,13,30,35 hypophosphatemia, raised liver enzymes and decreased renal function.15 Although CRP was markedly high, 51% of the patients did not have raised white cell count or neutrophilia, which again, was found by Sopena,3 Viasus30 and Benito.32
In 25.5% of patients, there was a platelet count below 171×109/L, which is one of the independent predictor variables in the LD score proposed by Fiumefreddo et al.13
The majority of patients had hypoxemia (PO2 <70mmHg), with 30.2% having PO2 <60mmHg, findings that are similar to those found by Benito et al.,32 with 33% and Sopena et al.3 with 31%. It is unusual to find any references in the literature to PCO2 values but our series showed that PCO2 was below 35mmHg in 93% of patients and this hypocapnia could be an indirect marker of tachypnea.
Twenty-three percent of cases showed worsening of radiological findings during the first 72h of admission. This has been described in other studies36 suggesting that it could be a more particular feature of LD than of other pneumonias. Half of these patients needed admission to the ICU and two required IMV.
Despite being highly sensitive, a sensitivity close to 100% according to Rihs et al.,37 the UAT for L. pneumophila serogroup 1 (Alere BinaxNOW®Legionella Urinary Antigen Card), which was applied to all our patients, was positive in 83.7%. An even smaller number was found in the Murcia outbreak, with only 47.7% of the cases having positive UAT. The variability of sensitivity could reflect disease severity; with higher positive rates in more severe disease.38 Our data also questions the indication for blood cultures during LD outbreaks.
Finally, in our series all patients were treated with high dose levofloxacin in monotherapy and there were no deaths. However, alertness was high because the outbreak had already been declared when our patients were admitted (after 7th November 2014). To evaluate case fatality rate, the DGH included deaths occurring since 1st October 2014 and a few patients died within this period.8
In our series early diagnosis, prompt treatment and optimization of process of care may have contributed to these results.
ConclusionsAlthough clinical presentation is not specific, our study shows that even during an outbreak, the combination of risk factors, symptoms and laboratory findings can be highly suggestive of LD. This can and should prompt further investigations in order to confirm LD, particularly in patients with less severe presentation or with negative UAT. Also, widespread use of UAT, not limited to the severe cases of community acquired pneumonia with ICU admission criteria, might contribute to earlier diagnosis, both in sporadic cases and in outbreaks of LD.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to thank Dr. Francisco George, Director-General of the Portuguese Directorate General of Health.