Rapid on-site evaluation (ROSE) has the potential to increase endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) accuracy in the diagnosis of mediastinal lesions and lung cancer staging. However, studies have reported controversial results.
The purpose of our study was to evaluate the influence of ROSE on sample adequacy and diagnostic accuracy of EBUS-TBNA.
MethodsProspective observational study that enrolled 81 patients who underwent EBUS-TBNA for investigation of hilo-mediastinal lesions or lung cancer staging. The first 41 patients underwent EBUS-TBNA with ROSE (ROSE group) and the last 40 patients without ROSE (non-ROSE group). Sample adequacy and diagnostic accuracy of EBUS-TBNA in both groups were compared.
ResultsAdequate samples were obtained in 93% of the patients in the ROSE group and 80% in non-ROSE group (p=0.10). The diagnostic accuracy of EBUS-TBNA was 91% in ROSE group and 83% in non-ROSE group (p=0.08). Analyzing the EBUS-TBNA purpose, in the subgroup of patients who underwent EBUS-TBNA for investigation of hilo-mediastinal lesions, these differences between ROSE and non-ROSE group were higher compared to lung cancer staging, 93% of patients with adequate samples in the ROSE group vs. 75% in the non-ROSE group (p=0.06) and 87% of diagnostic accuracy in ROSE group vs. 77% in non-ROSE group (p=0.10).
ConclusionsDespite the lack of statistical significance, ROSE appears to be particularly useful in the diagnostic work-up of hilo-mediastinal lesions, increasing the diagnostic yield of EBUS-TBNA.
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a technique that allows performing on “real time” TBNA, under ultrasound imaging. It is a minimally invasive procedure that was initially developed for lymph node staging of patients with non-small cell lung carcinoma and its use was rapidly extended for investigation of hilo-mediastinal adenopathy and masses.1–3
Rapid on-site evaluation (ROSE) during EBUS-TBNA allows the assessment of the adequacy of the samples and whether there is sufficient material for definitive pathological diagnosis.1,4 Therefore ROSE has the potential to increase the diagnostic yield of EBUS-TBNA.
Studies have reported a low rate of non-diagnostic sampling, a high agreement between ROSE and definitive pathological diagnosis as well as a decrease in the number of punctures and in the procedure time, with ROSE.5–7 However, the advantage of ROSE has been questioned because there is no remarkable difference in diagnostic yield between EBUS-TBNA results with or without ROSE.7–9 Thus, the role of ROSE during EBUS-TBNA remains controversial.
In this study we evaluated the clinical impact of the availability of ROSE in EBUS-TBNA results. Primary endpoints were to compare the adequacy of the samples and the diagnostic yield of EBUS-TBNA, performed with and without ROSE, in the diagnosis of hilo-mediastinal lesions and lung cancer staging. Secondary endpoints were the evaluation of the number of punctures per exam and the complication rate, with and without ROSE.
Materials and methodsPatientsOver a 1-year period, from January to December 2012, 81 consecutive patients underwent EBUS-TBNA for lung cancer staging or diagnosis of hilo-mediastinal lesions in the Bronchology Department of Centro Hospitalar de São João.
A prospective cohort observational study was done. The first 41 patients underwent EBUS-TBNA with ROSE (ROSE group) and the last 40 patients without ROSE (non-ROSE group).
EBUS-TBNA database was prospectively fulfilled and includes several variables for each patient: demographic characteristics, objective of EBUS-TBNA (investigation of hilo-mediastinal lesion or lung cancer staging), type of biopsied lesion (lymph node and/or mass), diameter of the lesions, number of punctures per procedure, complication rate, pathological results and microbiological analysis of EBUS-TBNA samples. Clinical files were also analyzed for this study.
The study was approved by the ethical committee of Centro Hospitalar de São João. Being an observational study, additional informed consent was not obtained.
Rapid on-site evaluation (ROSE)Until August 2012, ROSE was available for all patients (ROSE group). All the aspirated material was smeared onto glass slides, air dried or fixed in 95% alcohol and stained with May-Grunwald-Giemsa or hematoxylin eosin, respectively, for cytological evaluation. In the last puncture, needle was washed in a phosphate-buffered sucrose (PBS) solution, including the remaining material in the needle with more probability of blood clot, to perform subsequently a cytoblock for further ancillary techniques. The sample was considered adequate if lymphocytes (at least 40 lymphocytes in a high-power field1) or antracosis were identified or when a specific diagnosis was performed. When no specific diagnosis was reached during ROSE, a minimum of four punctures per lymph node was taken. The definitive pathological diagnosis considered was the one achieved after the observation of the entire sample. Immunocytochemical stains were done as needed.
From August 2012 until the end of the year, ROSE was not available and the whole sample was washed on the PBS solution and later included in paraffin block for diagnosis and further molecular studies. In this period, at least four punctures per lesion were taken in all the patients (non-ROSE group).
Samples obtained were classified as: (1) malignant; (2) specific benign diagnosis (e.g. sarcoidosis); (3) reactive lymphoid tissue; (4) inconclusive (e.g. suspicion of malignancy) and (5) non-representative. These two last types of samples were defined as non-diagnostic.
EBUS-TBNAAll procedures were performed under general anesthesia using the rigid bronchoscope. EBUS-TBNA was performed through a dedicated endoscope (BF-UC180F, Olympus) using a convex probe with a 7.5mHz transducer coupled on its tip which provides images parallel to the insertion of the bronchoscope. A 22-gauge needle (NA-201SX-4022, Olympus) was used to perform the aspirations.
The exams were carried out by the same three pulmonologists with about 2 years of experience in performing EBUS-TBNA, who had at least performed 40 or more procedures.
Final diagnosisThe final diagnosis was based on EBUS-TBNA definitive pathological and microbiological results. In case of reactive lymphoid tissue or non-diagnostic samples, the final diagnosis was established or confirmed through additional exams and/or by clinical and radiographic follow-up for at least 6 months.
Statistical analysisData were analyzed with IBM® SPSS® Statistics version 19.0 software. We reported data for continuous variables as means (with standard deviation) and for categorical variables as percentages. Unpaired t Student's test was used to compare age, diameter of lesions and number of punctures between two groups. Chi square test or Fisher's exact test were used to compare results of EBUS-TBNA (adequacy of the aspirated material and diagnostic accuracy) in the two groups. Results were considered statistically significant when the p value was less than 0.05. Diagnostic sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy were determined according to the standard definitions. We calculated sensitivity, NPV and accuracy with and without inclusion of non-diagnostic specimens (adding to false negative samples). Test performance measures were calculated as a combination of all samples per patient.
ResultsBetween January and December 2012, 81 patients who performed EBUS-TBNA were divided into two groups: 41 patients in ROSE group and 40 patients in non-ROSE group.
The indication for EBUS-TBNA was the investigation of hilo-mediastinal lesions in 29 patients (71%) in ROSE group and in 32 patients (80%) in non-ROSE group; and lung cancer staging in 12 patients (29%) in ROSE group and in 8 patients (20%) in non-ROSE group.
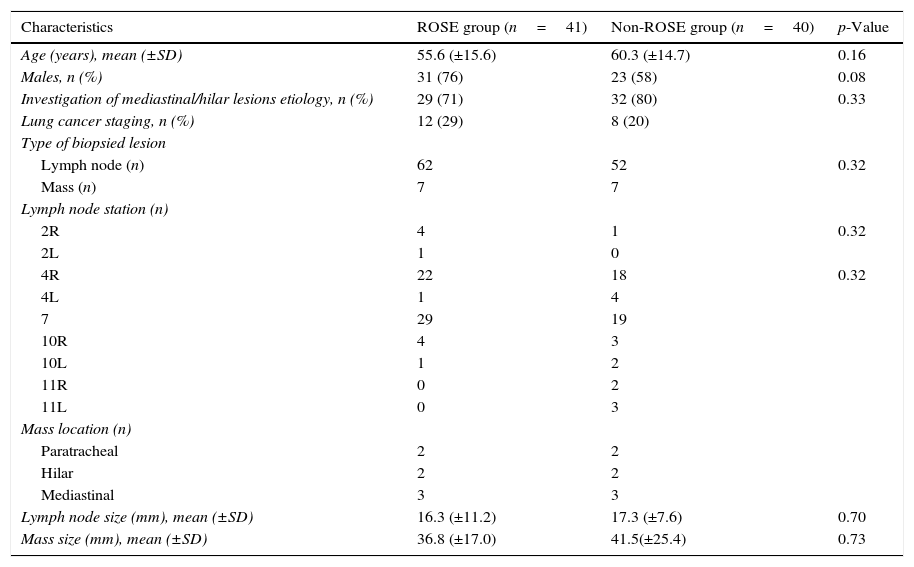
There was no statistically significant difference between the groups in respect to age, gender, type and diameter of the lesion (Table 1).
Demographic characteristics and baseline data.
| Characteristics | ROSE group (n=41) | Non-ROSE group (n=40) | p-Value |
|---|---|---|---|
| Age (years), mean (±SD) | 55.6 (±15.6) | 60.3 (±14.7) | 0.16 |
| Males, n (%) | 31 (76) | 23 (58) | 0.08 |
| Investigation of mediastinal/hilar lesions etiology, n (%) | 29 (71) | 32 (80) | 0.33 |
| Lung cancer staging, n (%) | 12 (29) | 8 (20) | |
| Type of biopsied lesion | |||
| Lymph node (n) | 62 | 52 | 0.32 |
| Mass (n) | 7 | 7 | |
| Lymph node station (n) | |||
| 2R | 4 | 1 | 0.32 |
| 2L | 1 | 0 | |
| 4R | 22 | 18 | 0.32 |
| 4L | 1 | 4 | |
| 7 | 29 | 19 | |
| 10R | 4 | 3 | |
| 10L | 1 | 2 | |
| 11R | 0 | 2 | |
| 11L | 0 | 3 | |
| Mass location (n) | |||
| Paratracheal | 2 | 2 | |
| Hilar | 2 | 2 | |
| Mediastinal | 3 | 3 | |
| Lymph node size (mm), mean (±SD) | 16.3 (±11.2) | 17.3 (±7.6) | 0.70 |
| Mass size (mm), mean (±SD) | 36.8 (±17.0) | 41.5(±25.4) | 0.73 |
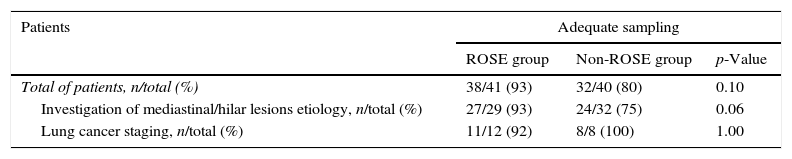
The percentage of patients with adequate samples was 93% in ROSE group and 80% in non-ROSE group (p=0.10).
When the objective of the procedure was investigation of hilo-mediastinal lesions, 93% of patients had adequate samples in ROSE group and 75% in non-ROSE group (p=0.06). When the purpose was lung cancer staging, 92% patients had adequate samples in ROSE group and 100% in non-ROSE group (p=1.00) (Table 2).
Adequacy of cytological samples.
| Patients | Adequate sampling | ||
|---|---|---|---|
| ROSE group | Non-ROSE group | p-Value | |
| Total of patients, n/total (%) | 38/41 (93) | 32/40 (80) | 0.10 |
| Investigation of mediastinal/hilar lesions etiology, n/total (%) | 27/29 (93) | 24/32 (75) | 0.06 |
| Lung cancer staging, n/total (%) | 11/12 (92) | 8/8 (100) | 1.00 |
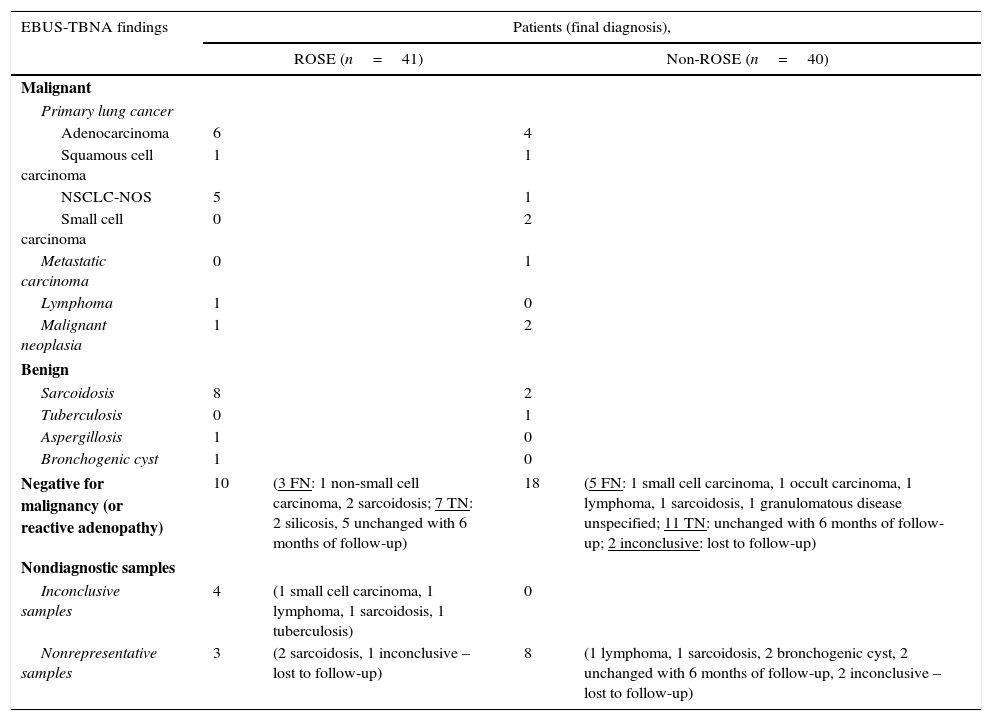
EBUS-TBNA results and the final diagnosis in both groups are described in Table 3. Reactive lymphoid tissue or non-diagnostic samples occurred in 17 of 41 patients (42%) in ROSE group and in 26 of 40 patients (65%) in non-ROSE group. These patients underwent additional diagnostic procedures and/or were followed up clinically and radiographically at least during 6 months to establish the final diagnosis. In ROSE group, the final diagnosis was based on additional procedures in five patients: two transthoracic needle aspiration, one thoracotomy, one mediastinoscopy and one bronchoalveolar lavage; diagnosis of the remaining patients was based on follow-up (except one patient – lost to follow-up). In the non-ROSE group, additional tests were required in 14 patients: four thoracotomy, two peripheral lymph node biopsy, two EBUS-TBNA repetition, one transthoracic needle aspiration, one pleural biopsy, one bronchial biopsy, two bronchoalveolar lavage, and one mediastinoscopy; clinical and radiographical follow-up established the final diagnosis in eight patients; the remaining four patients were lost to follow-up.
Results of EBUS-TBNA and final diagnosis.
| EBUS-TBNA findings | Patients (final diagnosis), | |||
|---|---|---|---|---|
| ROSE (n=41) | Non-ROSE (n=40) | |||
| Malignant | ||||
| Primary lung cancer | ||||
| Adenocarcinoma | 6 | 4 | ||
| Squamous cell carcinoma | 1 | 1 | ||
| NSCLC-NOS | 5 | 1 | ||
| Small cell carcinoma | 0 | 2 | ||
| Metastatic carcinoma | 0 | 1 | ||
| Lymphoma | 1 | 0 | ||
| Malignant neoplasia | 1 | 2 | ||
| Benign | ||||
| Sarcoidosis | 8 | 2 | ||
| Tuberculosis | 0 | 1 | ||
| Aspergillosis | 1 | 0 | ||
| Bronchogenic cyst | 1 | 0 | ||
| Negative for malignancy (or reactive adenopathy) | 10 | (3 FN: 1 non-small cell carcinoma, 2 sarcoidosis; 7 TN: 2 silicosis, 5 unchanged with 6 months of follow-up) | 18 | (5 FN: 1 small cell carcinoma, 1 occult carcinoma, 1 lymphoma, 1 sarcoidosis, 1 granulomatous disease unspecified; 11 TN: unchanged with 6 months of follow-up; 2 inconclusive: lost to follow-up) |
| Nondiagnostic samples | ||||
| Inconclusive samples | 4 | (1 small cell carcinoma, 1 lymphoma, 1 sarcoidosis, 1 tuberculosis) | 0 | |
| Nonrepresentative samples | 3 | (2 sarcoidosis, 1 inconclusive – lost to follow-up) | 8 | (1 lymphoma, 1 sarcoidosis, 2 bronchogenic cyst, 2 unchanged with 6 months of follow-up, 2 inconclusive – lost to follow-up) |
NSCLC-NOS, non-small cell lung carcinoma not otherwise specified; FN, false negatives; TN, true negatives.
In the ROSE group, on-site evaluation was discordant with definitive pathological diagnosis in four cases (10%). Disagreement was due to ROSE sample misinterpretation; the abnormality was not identified on-site, but after the observation of the whole sample.
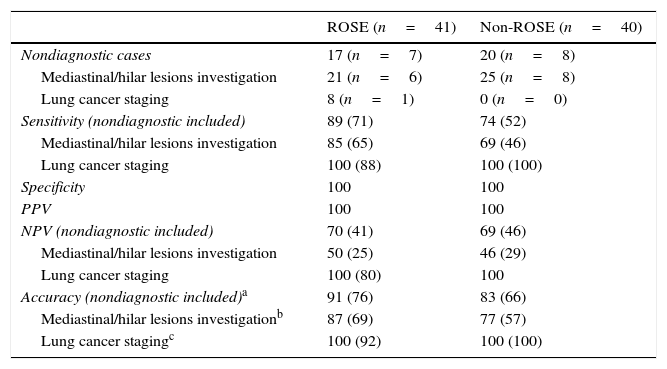
The overall diagnostic performance of EBUS-TBNA per patient with and without ROSE is detailed in Table 4. Two patients of non-ROSE group, with a diagnostic of reactive lymphoid tissue by EBUS-TBNA and lost to follow-up, were excluded. The diagnostic accuracy was 91% in the ROSE group and 83% in the non-ROSE group (p=0.08). There were seven and eight patients in ROSE and non-ROSE groups, respectively, with non-diagnostic samples. Adding the non-diagnostic samples to false negative samples, diagnostic accuracy decreased to 76% in ROSE group and 66% in non-ROSE group (p=0.20).
Diagnostic performance of EBUS-TBNA with and without ROSE.
| ROSE (n=41) | Non-ROSE (n=40) | |
|---|---|---|
| Nondiagnostic cases | 17 (n=7) | 20 (n=8) |
| Mediastinal/hilar lesions investigation | 21 (n=6) | 25 (n=8) |
| Lung cancer staging | 8 (n=1) | 0 (n=0) |
| Sensitivity (nondiagnostic included) | 89 (71) | 74 (52) |
| Mediastinal/hilar lesions investigation | 85 (65) | 69 (46) |
| Lung cancer staging | 100 (88) | 100 (100) |
| Specificity | 100 | 100 |
| PPV | 100 | 100 |
| NPV (nondiagnostic included) | 70 (41) | 69 (46) |
| Mediastinal/hilar lesions investigation | 50 (25) | 46 (29) |
| Lung cancer staging | 100 (80) | 100 |
| Accuracy (nondiagnostic included)a | 91 (76) | 83 (66) |
| Mediastinal/hilar lesions investigationb | 87 (69) | 77 (57) |
| Lung cancer stagingc | 100 (92) | 100 (100) |
Data are presented as %.
Performance measures of EBUS-TBNA according to the indication of the exam were separately calculated (Table 4): for hilo-mediastinal lesions investigation, diagnostic accuracy of EBUS-TBNA was 87% in ROSE group vs. 77% in non-ROSE group (p=0.10); for lung cancer staging diagnostic accuracy was 100% in both groups.
The number of punctures per procedure was significantly lower in the ROSE group (3.4±1.7 vs. 4.5±1.7 punctures, p=0.002).
In both groups there were no complications.
DiscussionOne of the main concerns of EBUS-TBNA is the quality of the sample obtained. ROSE allows the immediate confirmation of the presence of lymph node tissue or the acquisition of a specific diagnosis and, at the same time, helps selecting additional studies such as immunocytochemistry, microbiology, flow cytometry and molecular studies. The question is if this information substantially contributes to increase diagnostic accuracy of the test.
In this study, we found no statistically significant difference between the ROSE group and non-ROSE group in terms of sample adequacy. However, we found an important trend toward having more adequate samples with ROSE in patients for whom the purpose of the exam was the study of hilo-mediastinal lesions (93% of patients with adequate samples in ROSE group vs. 75% in non-ROSE group, p=0.06).
In the non-ROSE group, sample adequacy might have been improved by the performance of, at least, four punctures per lesion. Studies have reported that three aspirates per lesion maximized the yield of EBUS-TBNA for the staging of lung cancer.10 For the acquisition of high-quality tissue for molecular profiling for lung cancer genotyping, Yarmus et al.11 showed that a minimum of four needle passes per lesion were needed.
Regarding diagnostic accuracy, despite the lack of statistical significance, it was higher in ROSE group than in non-ROSE group in the investigation of hilo-mediastinal lesions (87% in ROSE group vs. 77% in non-ROSE group, p=0.10). Previous studies have suggested that on-site evaluation of TBNA specimens increase the diagnostic yield.12 Gu et al.,13 in a meta-analysis, also found this tendency to get better results with ROSE, but the heterogeneity between the studies hampered obtaining satisfactory results. On the other hand, Oki et al.,7 in a randomized study, and Griffin et al.,8 in a retrospective study, found that ROSE did not significantly increase the diagnostic yield of EBUS-TBNA. Concerning conventional TBNA, recent randomized studies of Trisolini et al.14 and Yarmus et al.15 also showed no benefit on diagnostic yield related to the use of ROSE.
Relatively to lung cancer staging, we did not find advantages of ROSE either in sample adequacy or in diagnostic accuracy. Therefore, in this study, ROSE was more useful for mediastinal lesions diagnosis than for lung cancer staging. The lack of benefit of ROSE in lung cancer staging could be explained by the small sample size of this subgroup of patients. Consequently, these results need to be confirmed in larger samples.
To achieve the final diagnosis, the patients of non-ROSE group needed more additional procedures. However, this fact can be justified by the higher prevalence of reactive lymph nodes in this group that required additional investigation.
ROSE during EBUS-TBNA decreased the number of punctures per procedure. However, the increase in the number of punctures in non-ROSE group was not associated to a higher complication rate. These results are in accordance with other publications.6,7
This study enrolled an unselected group of consecutive patients, with various diagnostic suspicions, who performed EBUS-TBNA. Therefore, it confirms the usefulness of EBUS-TBNA as the initial test not only for lung cancer diagnosis or staging but also for investigation of hilo-mediastinal lesions observed in computerized tomography (e.g. in the diagnosis of sarcoidosis).
Some limitations of this study have to be addressed. The sample was heterogeneous and too small to demonstrate, unequivocally, the advantage of ROSE regarding adequacy of samples and diagnostic accuracy of EBUS-TBNA. Therefore, no statistical significant difference between the two groups was achieved. There only was a trend to obtain better results in ROSE group when the purpose was the diagnosis of hilo-mediastinal lesions. Being an observational study, some confounding variables could not be controlled, such as the fact that ROSE group had been evaluated before the non-ROSE group and the interventional pneumologists had acquired more experience.
ConclusionThe adequacy of the samples and the diagnostic accuracy of EBUS-TBNA were not statistically influenced by the availability of ROSE. Despite this, there was a trend toward achieving more adequate samples and better diagnostic accuracy with ROSE in the investigation of hilo-mediastinal lesions. For lung cancer staging this advantage was not evident.
Conflict of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
AuthorshipAll listed authors participated in the study conception and design, in the data acquisition/analysis and in the writing of the article.