Transfusion-related Acute Lung Injury (TRALI) is a serious and potentially fatal complication of blood product administration, in which the patient develops Acute Respiratory Distress Syndrome (ARDS) with rapid-onset lung injury and non-cardiogenic pulmonary edema due to activation of immune cells in the lungs.1
The first series of cases using the term TRALI was published in 1985.2 However, the first definition and standardized criteria were only achieved at the Canadian Consensus Conference in 2004.3 Fifteen years later (2019), based on new knowledge gained since, a modified classification using the Revised Delphi panel definition method was proposed.4 In this classification, TRALI is considered as acute onset hypoxemia (PaO2/FiO2 ≤ 300 or SpO2 < 90 % on room air) and clear evidence of bilateral pulmonary edema on imaging (chest radiograph, computed tomography or ultrasound) during or within 6 h of a transfusion. No evidence of left atrial hypertension (LAH) should be present or, if LAH is present, it is judged not to be the main contributor to the hypoxemia.
Moreover, TRALI can be classified as TRALI type I in patients without concomitant risk factors for ARDS or TRALI type II in patients with risk factors or previous mild ARDS if stable respiratory status in the 12 h before transfusion. 4
TRALI is thought to involve a “two-hit” pathophysiology in which the first event is the underlying condition of the patient and the second event is the transfusion.5 Therefore, risk factors can be divided as recipient risk factors and blood component risk factors. Conditions historically associated with increased risk of TRALI include sepsis, noncardiogenic shock, massive transfusion, positive fluid balance, chronic alcohol abuse, current smoking, end-stage liver disease, liver transplantation surgery, thrombotic microangiopathy, and hematologic malignancy.4,6
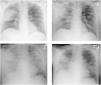
We present the case of a 48-years-old Caucasian male, with a personal history of chronic alcohol abuse and current smoker (20 UMA). No history of respiratory diseases was known. The patient was admitted to the emergency department with a three-day history of fever, dyspnea and cough with mucopurulent sputum. During physical examination, he was conscious but disoriented, tachycardic and tachypneic, with a hypotensive profile. Pulmonary auscultation revealed a decrease in the breath sounds over the right middle hemithorax. Arterial blood gas analysis revealed partial respiratory failure and hyperlactatemia (lactate of 7.7 mmol/L). Laboratory tests showed thrombocytopenia, acute kidney injury and liver failure. Urinary antigen was positive for Streptococcus pneumonia. Chest radiography showed an opacification in the middle level of the right hemithorax (Fig. 1) and computed tomography of the chest revealed a consolidation in the posterior segment of the right upper lobe.
In the first 24 h after admission, there was a worsening of the state of consciousness, hypotension and deteriorating respiratory failure. The diagnosis of community-acquired pneumonia due to Streptococcus pneumoniae complicated by septic shock with multiorgan dysfunction (respiratory, neurological, cardiovascular, hematological and hepatic) was established and the patient was admitted to the Intensive Care Unit.
The patient maintained an unfavorable evolution with hypoxic respiratory failure (PaO2/FiO2 = 107) requiring invasive mechanical ventilation, which he maintained for 7 days, initially with volume control and later with pressure support ventilation. Afterwards, there was slow clinical, laboratory and imaging improvement.
On the 13th day of hospitalization, he was hemodynamically stable and eupneic with oxygen therapy (FiO2 = 0.36, PaO2/FiO2 = 208, Table 1). Due to acute anemia, two units of erythrocyte concentrate were given to the patient. After completing the transfusion, there was worsening of the hypoxemia (PaO2/FiO2 = 120, Table 1) and pulmonary auscultation revealed disperse rales, especially bibasilar. Chest radiography showed new bilateral pulmonary infiltrates (Fig. 1).
Blood gas analyses before the transfusion, after the onset of TRALI and after treatment for TRALI was started.
PaO2 – arterial pressure of oxygen; PaCO2 – arterial pressure of carbon dioxide; FiO2 - fraction of inspired oxygen; NIV - non-invasive mechanical ventilation; IPAP - inspiratory positive airway pressure; EPAP - expiratory positive airway pressure; RR - respiratory rate; BPM – breaths per minute.
He maintained fever, which was difficult to assess due to concomitant prostatitis, with normal procalcitonin. Vascular accesses were changed. An echocardiogram showed no signs of fluid overload, LAH or endocarditis. Videobronchoscopy was also performed with bronchoalveolar lavage, mycological, bacteriological examination and Pneumocystis jirovecii polymerase chain reaction, all negative. After the patient was stabilized, a chest computed tomography maintained diffuse bilateral confluent air space opacities and excluded local complications.
Given the suspicion of TRALI type II and exclusion of other causes of ARDS, non-invasive mechanical ventilation was started with bilevel ventilation and the dose of intravenous corticosteroid therapy was increased. The patient tolerated periods of high flow nasal cannula alternating with bilevel ventilation the following day and subsequently maintained favorable clinical and radiological evolution. He was later discharged without need for supplementary oxygen.
In the case described, the patient initially presented with severe respiratory failure due to Pneumococcal Pneumonia but was evolving favorably until a sudden aggravation 6 h after a transfusion. Extensive investigation did not show any other probable causes for ARDS. Risk factors for TRALI, such as chronic alcohol abuse and smoking, were also present in this case.
In conclusion, TRALI is a rare and underdiagnosed adverse transfusional effect mostly because it is not always possible to exclude other causes of ARDS. In the presence of acute or aggravated hypoxemia and diffuse bilateral pulmonary opacities up to 6 h after a transfusion, the possibility of TRALI should be considered. It is essential to include TRALI in the differential diagnosis of ARDS in order to guarantee appropriate treatment, especially in an Intensive Care setting where patients are likely to have multiple risk factors of high complexity.