Confirmation of tuberculosis (TB) in children is difficult, so clinicians use different procedures when deciding to treat.
ObjectiveIdentify criteria to initiate and maintain TB treatment in children younger than 5 years-old, without diagnosis confirmation.
DesignA web-based survey was distributed by email to the corresponding authors of journal articles on childhood TB. The observations were clustered into disjoint groups, and analyzed by Ward's method.
ResultsWe sent out 260 questionnaires and received 64 (24.6%) responses. Forty-six respondents (71.9%) said that microbiological confirmation was not important for initiation of anti-TB treatment, and that the epidemiological context and signs/symptoms suggestive of disease were most important. Sixty-one respondents (95.3%) said that the decision to continue therapy was mainly dependent on clinical improvement. A cluster of older respondents (median age: 52 years-old) who were active at a hospital or primary health care centre placed the most value on immunological test results and chest X-rays. A cluster of younger respondents (median age: 38 years-old) who were less experienced in management of TB placed more value on Interferon Gamma Release Assay (IGRA) results and chest computed tomography (CT) scans. A cluster of respondents with more experience in treating TB and working at specialized TB centres placed greater value on the clinical results and specific radiological alterations (“tree-in-bud” pattern and pleural effusion).
ConclusionTB management varied according to the age, work location and experience of the clinicians. It is necessary to establish standardized guidelines used for the diagnosis and decision to treat TB in children.
Childhood tuberculosis (TB) is a serious public health problem,1–3 and a consequence of poor control of TB in the adult population.3 Early diagnosis and initiation of therapy is crucial for effective TB control. Delayed diagnosis increases the risk of death and TB transmission in the community.4,5
In 2014, the World Health Organization (WHO) reported there were approximately 340,000 incident cases of TB among all countries in the European Region. Children under 15 years-old accounted for 3.9% of all cases, and children under 5 years-old accounted for 1.6% of all cases.1
Confirmation of a TB diagnosis by identification of the infectious agent can be difficult in children.6 In 2009, the rate of diagnostic confirmation among paediatric cases was only 19.2%.7 Sampling is particularly difficult in children under 10 years-old, and even if samples are obtained, the paucibacillary nature of the lesions may produce false-negative results.5,6,8–10 Thus, gastric lavage is frequently used for the diagnosis of TB in children under 6 years-old.6,10–12 Currently, clinicians consider clinical presentation, history of recent contact with infected individuals, immunological evidence of infection, radiological signs compatible with TB, and lack of clinical improvement following antibacterial treatment as indicators of TB, and for initiation of TB treatment.5,6,13,14 However, the variability and low specificity of clinical and radiological findings in children indicate that a diagnosis based on these criteria should be viewed with a high degree of suspicion.5,15
This study aims to identify the criteria in Europe that most frequently lead to the initiation and maintenance of empiric antibiotic treatment in children younger than 5 years-old with suspected TB, but without diagnostic confirmation. It is also examined the relationship of different characteristics of clinicians with varying attitudes towards the diagnosis and treatment of childhood TB.
MethodsThis study was based on the implementation of a web-based survey, through Google Drive, directed at doctors and researchers in Europe who had experience treating children with TB. This survey consisted of 28 multiple-choice and simple-answer questions, divided into 3 sections: (i) identification (age, gender, country, job title, locality of work, specialization, and years in the job); (ii) experience (years in TB and childhood TB, time spent in those areas, and number of patients with TB diagnoses); and (iii) diagnostic criteria. Several questions in this last section related to clinical experience with children under 5 years-old who had TB, but without confirmation of diagnosis, to identify the most important criteria used to start treatment and to differentiate the most important symptoms and results among radiological, immunological, and confirmation tests in these children. The main criteria used to maintain treatment, without TB confirmation, were also identified. All the responses were anonymous.
The names and addresses of the surveyed researchers were collected using software specifically developed for this purpose (described below). This software combines data from several sources, because we were unable to find a single source with data on research papers and their corresponding authors. The data sources were: PubMed, the Digital Object Identifier (DOI) System, and the web sites of journals that published the papers.
The collection process was driven by a web interface, where the user specified the keywords (“paediatric, tuberculosis”) and time interval (1 January 2005 to 20 December 2015). These data were submitted to a server, and processed in three stages. First, the PubMed database was queried using the Entrez Programming Utilities,16 which returned data on papers with the selected criteria (n=1573), and the title and DOI of each paper. PubMed does not record the corresponding authors, so this information was retrieved from the journal web sites. Second, the DOI name resolution service17 was used to obtain each paper's URL from its DOI. Third, using this URL, the paper's web page was retrieved from the journal's web site, and the name and email of the corresponding author was extracted (n=260), when this information was available.
Descriptive statistics (absolute and relative frequencies) are given for categorical variables, and medians, with minima and maxima, are given for quantitative variables. The chi-squared test (or Fisher's test, as adequate) evaluated the independence between two categorical variables while the Mann–Whitney (resp. the Kruskall–Wallis) test accessed the existence of significant differences between the distributions of two (resp. or a higher number of) independent quantitative variables.
All collected observations were clustered into disjoint groups according to responses in the “diagnostic criteria” section of the survey. As the variables were categorical, several agglomerative hierarchical clustering techniques, with a dissimilarity matrix given by the Gower distance, were used. The final clustering was from the method with the highest agglomerative coefficient (Ward's method). The division consisted of 4 clusters, one of which was eliminated because it only had 2 participants (outliers).
The statistical analyses were performed using R Language and Software Environment for Statistical Computation (version 3.3.0).18 The significance level was set at 0.05.
The definition of “confirmation of diagnosis” is a positive smear and nucleic acid-amplification test or positive culture for Mycobacterium tuberculosis.
The study was approved by the Ethics Committee of the EPIUnit – Institute of Public Health, University of Porto, Porto, Portugal.
ResultsThe overall response rate was 24.6% (64/260). The respondents had a median age of 46.5 years (range: 28–70), 54.7% (n=35) were female, and most were from European countries other than Portugal (78.1%; n=50). The greatest number of respondents were from Italy (15.6%; n=10), Turkey (10.9%; n=7), and the United Kingdom (9.4%; n=6). Thirty-nine respondents (60.9%) were medical doctors, 2 (3.1%) were researchers, and 23 (35.9%) were both doctors and researchers. Among the doctors, 26 (40.6%) were paediatricians, 19 (29.7%) were pneumologists, and 11 (17.2%) were infectious disease specialists. In addition, 13 of the doctors (20.3%) worked in specialized TB centres and 51 (79.7%) worked in a hospital or a primary care setting. TB accounted for more than half of the monthly workload for 15.6% (n=10) of the doctors, and infant TB accounted for more than one-quarter of the monthly workload for 25.0% (n=16) of the doctors. In 2014, the respondents diagnosed a median of 3 (range: 0–100) children younger than 5 years-old with TB, and a median of 2 (range: 0–50) had confirmed diagnoses of TB.
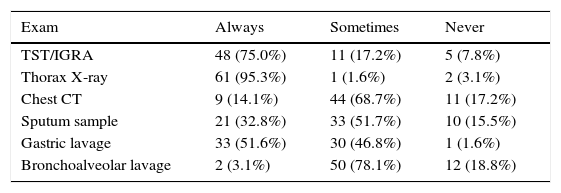
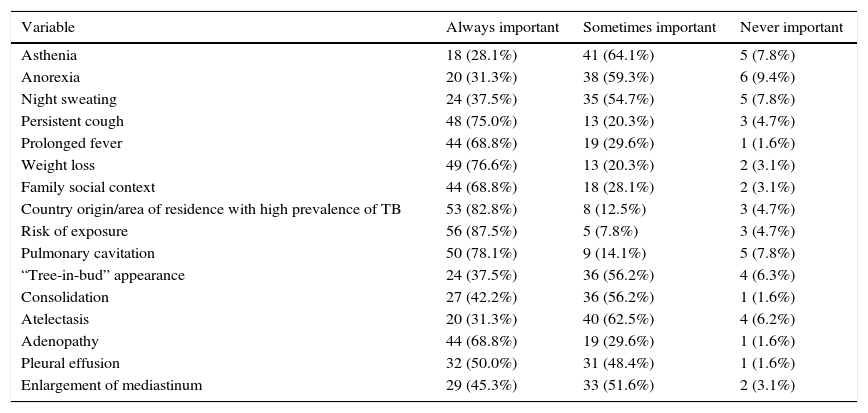
Forty-six respondents (71.9%) reported that microbiological confirmation was not important for their decisions to initiate anti-TB treatment. Respondents particularly valued the epidemiological context (89.1%; n=57), signs and symptoms suggestive of disease (85.9%; n=55), radiological findings (76.6%; n=49), patient age (48.4%; n=31), and results of the tuberculin skin test (TST)/interferon gamma release assay (IGRA) (45.3%; n=29). The most frequently requested exams following suspicion of TB were chest X-ray (95.3%; n=61), which was considered more important (78.3%; n=47) than a chest CT scan (21.7%; n=13). The respondents also placed great importance on the TST/IGRA results (75%; n=48), and the TST was considered more important (56.3%; n=27) than the IGRA (43.8%; n=21). Regarding sample collection, gastric lavage was the most commonly requested method (51.6%; n=33), followed by collection of sputum (32.8%; n=21) (Table 1). The respondents reported the most valued determinants for starting treatment without diagnostic confirmation were weight loss (76.6%; n=49), persistent cough (75.0%; n=48) and prolonged fever (68.8%; n=44), especially if they lasted more than 2 weeks (65.6%; n=42), history of exposure to TB (87.5%; n=56), high TB prevalence in the country or area of residence (82.8%; n=53), and radiological test results (93.8%; n=60), mainly cavitations (78.1%; n=47) and adenopathies (68.8%; n=44) (Table 2).
Exams most requested by survey respondents (n=64) during their clinical investigation of children under 5 years-old with suspected TB, but without diagnostic confirmation.
| Exam | Always | Sometimes | Never |
|---|---|---|---|
| TST/IGRA | 48 (75.0%) | 11 (17.2%) | 5 (7.8%) |
| Thorax X-ray | 61 (95.3%) | 1 (1.6%) | 2 (3.1%) |
| Chest CT | 9 (14.1%) | 44 (68.7%) | 11 (17.2%) |
| Sputum sample | 21 (32.8%) | 33 (51.7%) | 10 (15.5%) |
| Gastric lavage | 33 (51.6%) | 30 (46.8%) | 1 (1.6%) |
| Bronchoalveolar lavage | 2 (3.1%) | 50 (78.1%) | 12 (18.8%) |
Importance that survey respondents (n=64) assigned to different variables for initiation of anti-bacillary treatment in children under 5 years-old with suspected TB, but without diagnostic confirmation.
| Variable | Always important | Sometimes important | Never important |
|---|---|---|---|
| Asthenia | 18 (28.1%) | 41 (64.1%) | 5 (7.8%) |
| Anorexia | 20 (31.3%) | 38 (59.3%) | 6 (9.4%) |
| Night sweating | 24 (37.5%) | 35 (54.7%) | 5 (7.8%) |
| Persistent cough | 48 (75.0%) | 13 (20.3%) | 3 (4.7%) |
| Prolonged fever | 44 (68.8%) | 19 (29.6%) | 1 (1.6%) |
| Weight loss | 49 (76.6%) | 13 (20.3%) | 2 (3.1%) |
| Family social context | 44 (68.8%) | 18 (28.1%) | 2 (3.1%) |
| Country origin/area of residence with high prevalence of TB | 53 (82.8%) | 8 (12.5%) | 3 (4.7%) |
| Risk of exposure | 56 (87.5%) | 5 (7.8%) | 3 (4.7%) |
| Pulmonary cavitation | 50 (78.1%) | 9 (14.1%) | 5 (7.8%) |
| “Tree-in-bud” appearance | 24 (37.5%) | 36 (56.2%) | 4 (6.3%) |
| Consolidation | 27 (42.2%) | 36 (56.2%) | 1 (1.6%) |
| Atelectasis | 20 (31.3%) | 40 (62.5%) | 4 (6.2%) |
| Adenopathy | 44 (68.8%) | 19 (29.6%) | 1 (1.6%) |
| Pleural effusion | 32 (50.0%) | 31 (48.4%) | 1 (1.6%) |
| Enlargement of mediastinum | 29 (45.3%) | 33 (51.6%) | 2 (3.1%) |
A total of 95.3% of the respondents reported that maintenance of therapy, despite no confirmation of diagnosis, was mainly dependent on clinical improvement (85.3%; n=52), radiological improvement (68.9%; n=42), and the presence an immunosuppressed state (59.0%; n=36).
Cluster 1 consisted of older respondents (median age: 52 years-old) who worked in a hospital or primary health care centre (92.3%; n=24). These respondents placed most value on the immunological test results (88.5%; n=23), especially the TST (76.9%; n=20) rather than the IGRA, and the chest X-ray (95.8%; n=23) rather than the CT scan. Cluster 3 consisted of younger respondents (median age: 38 years-old) who had less experience in the diagnosis of TB in children (median children diagnosed with TB in 2014: 0; median children with confirmed diagnosis in 2014: 1). These respondents placed most value on the clinical findings (100%; n=17), the IGRA test (100%; n=17) rather than the TST, and the chest CT scan (33.3%; n=5) rather than the chest X-ray. Cluster 2 consisted of respondents who had more experience in the diagnosis of TB in children (median children diagnosed with TB in 2014: 8; median children with confirmed TB diagnosis in 2014: 3) and worked in specialized TB centres (36.8%; n=7). These respondents valued the clinical findings (89.5–100%; n=17–19) and specific radiological alterations, such as the “tree-in-bud” pattern (68.4%; n=13) and pleural effusion (79.0%; n=15).
DiscussionA total of 71.9% of the respondents reported that microbiological confirmation was not important for their decisions to start antibiotic treatment for TB in a child younger than 5 years-old. The new guidelines for management of TB in children also state that a diagnosis of TB can be made without confirmation by culture, although they recommend cultures for all children with suspected pulmonary TB.19 Starke et al.20 recommended that samples should only be collected from children when the index case is unknown, the infection is drug-resistant, there is extra-pulmonary TB, or the diagnosis is uncertain.
When diagnosing TB without confirmation by culture, our respondents placed most value on the epidemiological context, signs and symptoms suggestive of disease, radiological findings, and results of the TST and IGRA. These results are similar to those of Marais et al.21,22 and Graham et al.13, who reported that diagnostic confirmation is difficult in the early stages of TB, and that consideration of recent contacts, immunological data, and radiological signs allow accurate diagnosis in most cases. Similarly, Sant’Anna et al.23,24 reported that culture confirmation is not necessary for the diagnosis of TB in children who had had contact with adults who had infectious TB, TST positivity, or clinical and/or radiological findings suggestive of TB.6
It is, therefore, necessary to develop guidelines for the diagnosis and treatment of TB in children, in the absence of diagnostic confirmation, based on the criteria mentioned above. Although several authors have proposed guidelines, they are not yet standardized, making comparisons difficult, and they also have not yet been validated.25 A “score system” for paediatric pulmonary TB in Brazil had a sensitivity of 89–98% and a specificity of 86–98%, without inclusion of bacteriological confirmation.23,24
Maintaining TB therapy without confirmation of diagnosis mainly relies upon clinical and radiological improvement, because response to anti-bacillary treatment supports a probable diagnosis of TB. However, Marais et al.26 identified children with persistent radiological signs and paroxysmal exacerbation of symptoms or signs after starting therapy, and concluded that these were not indications for changing therapy. This is because radiological improvement may occur several months after resolution of symptoms. Furthermore, after initiation of therapy, there can be immunological reconstitution phenomena with increased inflammatory responses, in addition to the release of toxins, and this may explain the exacerbation of symptoms/signs.26
Also, TB with a negative sputum culture is very likely to be paucibacillary, with a very low risk of acquiring drug resistance, and this also favours continuation of therapy despite no confirmation of diagnosis. Compliance with the complete treatment regimen is effective in these cases, due to the lower probability of adverse effects in children than adults.26
We identified three clusters of respondents according to age, experience, and clinical practice. On the one hand, our results showed that those with less clinical experience with TB valued the same factors as those with more experience (clinical and radiological abnormalities). On the other hand, respondents who were older and worked in primary care settings or hospitals placed greater importance on immunological test results, especially the TST. These tests have limited ability to distinguish latent TB from active TB3,9,27 and the sensitivity of TST is very low in children.3,28 Clinicians working in specialized TB centres gave less importance to immunological test results, and greater importance to clinical presentation and radiological images. Chen et al.29 showed that physicians who had less experience with TB had an increased duration of diagnosis, and Kamran Khan et al.30 showed that 16.5% of patients treated by inexperienced TB professionals died in the first year after diagnosis, compared with only 6.2% of patients treated by experienced TB professionals. These authors also identified the importance of differences regarding direct experience with TB, not simply in the area of specialization.30
A limitation of this study was the low response rate (24.6%). We identified names and contacts using software that searched published papers on TB in children during the last 10 years. It was not possible to determine if all questionnaires were received, if the email of the corresponding authors were still active, or if the corresponding authors were still professionally active. The only data we have from the correspondent is that he/she published a paper on TB in children and an email address. The characteristics of a respondent and years of experience were only available if the questionnaire was answered. However, our methodology allowed identification of 64 clinicians who made decisions about the management of TB in children, and these respondents had different experiences and used different strategies. Another possible weakness of our study is that the answers could not be validated, and bad practices could have been omitted. However, we believe that because the questionnaire was anonymous (we could not link the name and email to the questionnaire, and all respondents were informed of this), respondents were more likely to provide honest answers. The strength of this study is that it allowed clustering of answers according to profession, age, experience, and work location. Our results indicate there is a clear need to establish a wider base for designing guidelines used to diagnose and treat TB in children.
ConclusionsThe procedures used to diagnose and treat TB in children vary according to clinicians’ experience, work location, and age. There is a need for clinicians to use better guidelines and to improve the diagnosis and treatment of TB in young children.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
AuthorshipSofia Ramos: conceived the study and collaborated in all steps. Rita Gaio: performed statistical analysis and reviewed the manuscript. Fábio Ferreira: performed statistical analysis. José Paulo Leal: developed the methodology. Sara Martins: collaborated in all steps. João Vasco Santos: contributed to study design and reviewed the manuscript. Isabel Carvalho: reviewed the manuscript. Raquel Duarte: supervised all aspects of this work.
Conflicts of interestThe authors have no conflicts of interest to declare.
Rita Gaio and Fábio Ferreira were partially supported by CMUP (UID/MAT/00144/2013), which is funded by FCT (Portugal) with national (MEC) and European structural funds (FEDER), under the partnership agreement PT2020.