To compare the prevalence of unexplained pulmonary artery hypertension (PAH) in hemodialysis (HD) and peritoneal dialysis (PD) patients and to compare laboratory parameters between patients with unexplained PAH and those with normal pulmonary artery pressure (PAP).
MethodsWe retrospectively reviewed the medical records of 278 chronic HD and 145 chronic PD patients. Laboratory findings including hemoglobin, calcium, phosphorus, alkaline phosphatase, albumin, parathyroid hormone level, serum iron, total iron binding capacity, ferritin, creatinine and blood urea nitrogen were documented. The results of transthoracic Doppler echocardiography were used to determine the pulmonary artery pressure (PAP). PAH was defined as a systolic pulmonary artery pressure (SPAP) ≥35mmHg. To rule out secondary PAH, patients with cardiac disease, pulmonary disease, collagen vascular disease, volume overload at the time of echocardiography and positive human immunodeficiency virus test were excluded.
ResultsData from 34 patients in group HD and 32 individuals in group PD were analyzed. The median age of the study population was 57 (45–68) years. The median SPAP value in patients with PAH was 37.5 (35–45)mmHg. According to the echocardiographic findings, PAH was found in 14 (41.1%) patients of HD group and in 6 (18.7%) patients of PD group (P=0.04). The median serum iron and hemoglobin was significantly lower in patients with PAH compared to those in patients with normal PAP (P<0.05).
ConclusionUnexplained PAH seems to be more frequent in patients undergoing HD than patients in PD group. Moreover, hemoglobin and serum iron levels are lower in patients with PAH compared to those in normal PAP group.
Comparar a prevalência de hipertensão arterial pulmonar (PAH) inexplicável em doentes sob hemodiálise (HD) e diálise peritoneal (PD) e comparar os parâmetros laboratoriais entre doentes com PAH inexplicável e aqueles com pressão arterial pulmonar normal (PAP).
MétodosRevimos, de forma retrospetiva, os registos médicos de 278 doentes com HD crónica e 145 com PD crónica. Dos dados laboratoriais foram registadas hemoglobina, cálcio, fósforo, fosfatase alcalina, albumina, nível de paratormona, ferro sérico, capacidade total de ligação de ferro, ferritina, creatinina e nitrogénio ureico no sangue. Os resultados do ecocardiograma doppler transtorácico foram utilizados para determinar a pressão arterial pulmonar (PAP). A PAH foi definida como uma pressão arterial pulmonar sistólica (SPAP) ≥35mmHg. Para excluir a PAH secundária, foram excluídos os pacientes com problemas cardíacos, doenças pulmonares, doenças vasculares do colagénio, excesso de volume na altura do ecocardiograma e vírus de imunodeficiência humana positivo.
ResultadosForam analisados dados de 34 pacientes no HD e 32 indivíduos no grupo PD. A mediana de idade da população estudada foi de 57 (45–68) anos. O valor médio de SPAP em doentes com PAH foi de 37,5 (35–45) mmHg. De acordo com os resultados do ecocardiograma, a PAH foi registada em 14 (41,1%) pacientes do grupo HD e em 6 (18,7%) pacientes do grupo PD (P=0,04). A mediana do ferro sérico médio e da hemoglobina estavam significativamente mais baixos em pacientes com PAH em comparação com os pacientes com PAP normal (P<0,05).
ConclusãoA PAH inexplicável parece ser mais frequente em pacientes com HD do que em pacientes no grupo de PD. Além disso, os níveis de hemoglobina e ferro sérico são inferiores em pacientes com PAH comparando com os do grupo de PAP normal.
Pulmonary arterial hypertension (PAH) is a newly recognized disease in patients with renal disease.1 In clinical practice, shunting of blood from the left to the right side of the heart and increased cardiac output and pulmonary blood flow are common medical conditions resulting in PAH.2 However, Yigla et al. first noted unexplained PAH in some long-term hemodialysis (HD) patients during an epidemiologic study.3 They attributed both end stage renal disease (ESRD) and long-term HD therapy via an arteriovenous (AV) access to the pathogenesis of PAH in these patients.3,4 On the other hand, the prevalence of PAH in patients on peritoneal dialysis (PD) is still a matter of debate.5,6 The information in the literature regarding unexplained or primary PAH in ESRD patients especially PD patients is limited. Therefore, the aim of the present study was to compare the prevalence of unexplained PAH in HD and PD patients. In addition, we aimed to compare laboratory parameters between patients with unexplained PAH and those with normal pulmonary artery pressure (PAP).
Materials and methodsWe retrospectively reviewed the medical records of 278 chronic HD and 145 chronic PD patients treated at the hospitals affiliated to the university in Tabriz, Iran between May 2008 and January 2010. The patients’ data including age, sex, co-morbidities, medications, tobacco use, etiology of renal failure, vascular access type, and duration of dialysis therapy were recorded. Laboratory findings including hemoglobin, calcium, phosphorus, alkaline phosphatase, albumin, parathyroid hormone (PTH) level, serum iron, total iron binding capacity, ferritin, creatinine and blood urea nitrogen were documented. The results of transthoracic Doppler echocardiography were used to determine the pulmonary artery pressure, expiratory and inspiratory inferior vena cava (IVC) diameters and percent collapse, left ventricular ejection fraction, presence of valvular diseases, etc. PAH was defined as a systolic pulmonary artery pressure (SPAP) ≥35mmHg.
Patients with cardiac disease, pulmonary disease, collagen vascular disease, volume overload at the time of echocardiography (<50% collapsibility in IVC diameter) and positive human immunodeficiency virus (HIV) test were excluded. Additionally, patients treated with dialysis <3 months or >7 years were not included in the present study.
Data were presented as median (interquartile range). All statistical analyses were performed with Statistical Package of Social Science (SPSS Inc., Chicago, IL) for Windows version 16. The Mann–Whitney U test, chi-square test and Fisher's exact test were used wherever appropriate. A P-value <0.05 was considered statistically significant.
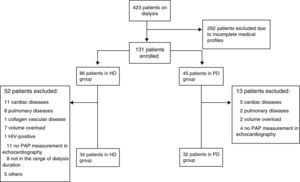
ResultsData from 66 patients were analyzed: 34 in group HD and 32 in group PD (Fig. 1). The median age of the study population was 57 (45–68) years. The median duration of dialysis was 102 (54.25–161) weeks in HD group and 44 (24.5–82) weeks in PD group (P=0.001, Mann–Whitney U test). Among HD group, 18 patients (53%) had proximal and 16 patients (47%) had distal arteriovenous fistula (AVF). However, none of the patients in PD group had functional AVF; 28 patients were primarily in PD group and 4 patients had primary nonfunctional AVF.
The median SPAP value in patients with PAH was 37.5 (35–45)mmHg. According to the echocardiographic findings, PAH was detected in 20 (30.3%) patients: 14 (41.1%) patients in HD group and 6 (18.7%) patients in PD group (P=0.04, Fisher's exact test). Among HD patients with PAH, vascular access type was proximal and distal AVF in 8 (57.1%) and 6 (42.9%) patients, respectively. None of the PD patients with PAH had vascular access. There were no differences in gender, age, weight, duration of dialysis, calcium, phosphorus, alkaline phosphatase, albumin, parathyroid hormone level, total iron binding capacity, ferritin, creatinine and blood urea nitrogen between the patients with PAH and those with normal PAP (Table 1, P>0.05). The median serum iron was significantly lower in patients with PAH (47μg/dL) compared to that in patients with normal PAP (71.5μg/dL, P=0.001, Table 1). The median hemoglobin of patients with normal PAP was not comparable to that of PAH patients (11.3mg/dL vs. 9.9mg/dL, P=0.004, Table 1).
Patients’ demographic data and laboratory parameters (n=number of patients).
| Normal PAP (n=46) | Elevated PAP (n=20) | P value | |
| Gender (male:female) | 22:24 | 8:12 | 0.79 |
| Age (years) | 53.5 (39.75–68.5) | 65 (53.75–67.75) | 0.14 |
| Weight (kg) | 66.5 (54–75) | 60.5 (53.25–69.5) | 0.21 |
| Duration of dialysis (weeks) | 57 (27.75–140) | 77 (44.5–171) | 0.32 |
| Hemoglobin (mg/dL) | 11.3 (9.95–12.05) | 9.9 (9.5–10.5) | 0.004* |
| Calcium (Ca) (mg/dL) | 8.8 (8.17–9.2) | 8.35 (7.82–9) | 0.20 |
| Phosphorus (P) (mg/dL) | 5.45 (4.37–6.7) | 6.11 (4.57–7.4) | 0.25 |
| Ca.P product | 46.2 (39.35–56.22) | 52.13 (38.2–61.37) | 0.45 |
| Alkaline phosphatase (IU/L) | 249 (193.75–297) | 215 (174–312.5) | 0.72 |
| Albumin (g/dL) | 4.1 (3.6–4.3) | 3.8 (3.62–4.2) | 0.24 |
| Parathyroid hormone (pg/mL) | 197 (71–412) | 213 (172–509) | 0.32 |
| Serum iron (SI) (μg/dL) | 71.5 (58.5–98.25) | 47 (31.5–63) | 0.001* |
| TIBC (μg/dL) | 291.5 (240.75–333) | 295 (231.5–329.5) | 0.98 |
| SI/TIBC | 0.25 (0.19–0.32) | 0.16 (0.14–0.21) | 0.002* |
| Ferritin (ng/mL) | 305 (148–682) | 410 (223.9–721) | 0.26 |
| Creatinine (mg/dL) | 7.75 (5.05–9.85) | 7.6 (6.57–9.07) | 0.77 |
| BUN (mg/dL) | 56 (45.12–77.37) | 70.5 (55.5–84.62) | 0.09 |
PAP, pulmonary artery pressure; TIBC, total iron binding capacity; BUN, blood urea nitrogen.
The present study revealed that PAH was more frequent in patients undergoing HD (41.1%) than in patients of PD group (18.7%). Previous studies have mostly reported the prevalence of PAH in HD patients, but not in PD patients. Amin et al.,7 Yigla et al.4 and Tarrass et al.8 concluded that there was a high prevalence of PAH among patients with ESRD receiving long-term HD with surgical AV access. The two latter studies differed with regard to the type of PAH; primary and secondary PAH in the former and primary PAH in the latter. Nakhoul and colleagues9 and Havlucu et al.10 found PAH in approximately half of the HD patients. Nakhoul et al. noted a significant decrease in PAP following successful kidney transplants and temporary closure of AV access in some PAH patients.9 Kumbar et al. detected PAH (primary and secondary) in 42% of patients on PD.5 In a recent study on HD and PD patients, Bozbas and co-workers found a higher PAH ratio in HD compared to that in the PD group (18.8% vs. 5.9%). Patients with secondary PAH were not excluded in their study.6 Furthermore, the duration of dialysis in the HD group was longer than that in the PD group. This may account for the differing incidence of PAH between the two groups. However, previous studies have highlighted no difference in the duration of HD therapy between patients with PAH and those without PAH.7,11
In recent studies numerous parameters have been compared between patients with PAH and those with normal PAP. In a study on HD patients, Abassi et al.12 and Yigla et al.4 found that those with elevated PAP had significantly lower hemoglobin and hematocrit levels. Similarly, we observed significantly lower hemoglobin levels in PAH patients in the present study. In contrast, other researchers have failed to detect any difference in hemoglobin and hematocrit levels between the two groups.5,6,9,10 In addition, we found lower serum iron levels in patients with PAH compared to those in normal PAP group. This finding has not been previously reported in patients undergoing HD and/or PD. In a recent study, Ruiter et al. interestingly revealed that iron deficiency was common in idiopathic PAH.13 The present study's findings are consistent with previous studies which found that PTH level and calcium–phosphorus product did not differ.4,5,7,9,12 However, Havlucu and colleagues reported higher calcium–phosphorus production and PTH levels in patients with PAH.10 Although we failed to find a significant difference in ferritin levels between PAH and normal PAP groups, Kumbar et al. disclosed lower ferritin levels in patients with PAH.5
There is not yet a clear explanation for the high prevalence of PAH in ESRD. In HD patients, numerous hormonal and metabolic disorders have been attributed to vasoconstriction of pulmonary vessels and pulmonary artery calcification.10,12,14 However, Amin et al.7 and Yigla et al.15 did not confirm the role of secondary hyperparathyroidism and pulmonary calcification as the etiology of PAH in ESRD patients. Later, Nakhoul et al. studied the role of two endothelial-derived molecules, endothelin-1 and nitric oxide (NO), in the pathogenesis of PAH in HD patients via AV access.9 Although impaired NO production and reduced sensitivity to NO have already been described in patients with chronic renal failure,16 Nakhoul et al. first demonstrated a link between impaired NO production and PAH in uremic patients receiving chronic HD therapy via AV access.9 This finding may be also generalized to the patients on PD therapy; NO deficiency has been described in PD patients.17 Along with high cardiac output resulting from the AV access, such an endothelial dysfunction may reduce the capability of the pulmonary vasculature to maintain the raised cardiac output and may subsequently contribute to the development of PAH in ESRD patients.9,18 An increase in the cardiac output and pulmonary blood flow due to the AV access may change shear stress.9 This may alter endothelial function or release of mediators through induction of gene expression patterns.19 Moreover, a venous torrent of microbubbles stemming from tubing or dialyzer has been proposed as a potential etiological factor in the development of PAH in this population. These microbubbles obstruct capillaries in the lungs, which may cause tissue ischemia, an inflammatory response and complement activation.20
This study has certain limitations. The retrospective observational design of the study might be considered a weakness in this work. Moreover, PAP was measured by a non-invasive method, Doppler echocardiography, without obtaining direct invasive measurements (e.g. right heart catheterization). However, measurements of PAP by the applied Doppler echocardiographic method have been reported to have a good correlation with measurements obtained by invasive methods in some studies.7,21,22 This method of PAP measurement has been widely applied in previous studies on PAH in patients on HD or PD.1,4–11,15 In addition, doses of erythropoiesis-stimulating agent (ESA) were not available in either group. Higher doses of ESA in either group might be a confounding factor and interesting to observe. Furthermore, the effect of the duration of dialysis on the incidence of PAH was not addressed in the present study.
In conclusion, unexplained PAH seems to be more frequent in patients undergoing HD than patients in the PD group. Moreover, among numerous laboratory parameters, hemoglobin and serum iron levels are lower in patients with PAH compared to those in normal PAP group. Due to the high importance of PAH in ESRD patients receiving both HD and PD therapy, the present findings need to be further explored in larger studies.
Conflicts of interestThe authors declare they have no conflicts of interest.