New-onset insomnia (NOI) associated with nocturnal ventilatory support (NVS) is becoming a reality in clinical practice; however there is a lack of data about its prevalence. Our aim was to determine the prevalence of NOI in patients with obstructive sleep apnoea syndrome (OSAS) under NVS and its associated risk factors.
Material and methodsDescriptive cross-sectional study of 80 patients with OSAS under NVS. We compared two groups, with and without NOI, considering demographic characteristics, disease features, and personality. Patients under anxiolytic and/or antidepressant medication, with a weight loss of 10% or greater, and with restless legs symptoms were excluded.
ResultsMedian age of patients was 60.0 (interquartile range (IQR) 10.0) years; 82.5% were male. Median initial Epworth Sleepiness Scale (ESS) and apnoea–hypopnoea index (AHI) were 12.5 (IQR 9.0) and 44.1 (IQR 22.4)/h, respectively. The majority of patients (91.3%) were under auto-adjusting positive airway pressure (APAP). Insomnia at baseline was present in 30% of patients (n=24). Prevalence of NOI was 21.4% (12/56). Initial and/or intermediate insomnia were the most frequent subtypes (n=11). We found a statistically significant negative relation between NOI and pressure on 90% nighttime (P90) (p=0.040).
ConclusionsOSAS patients under NVS presented a high prevalence of NOI. Patients with NOI presented lower levels of pressure using NVS, compared to the others.
A insónia de novo (IN) associada ao uso do suporte ventilatório nocturno (SVN) tem sido uma realidade constatada na prática clínica, contudo é de salientar a escassez de dados referentes à sua prevalência. O nosso objectivo consistiu em determinar a prevalência de IN e seus factores de risco em doentes com síndrome de apneia obstrutiva do sono (SAOS) sob SVN.
Material e métodosEstudo descritivo transversal que incluiu 80 doentes com SAOS sob SVN. Efectuada comparação entre dois grupos, com e sem IN, relativamente a características demográficas, relacionadas com a doença, e personalidade. Foram excluídos os doentes sob medicação ansiolítica e/ou antidepressiva, com perda ponderal superior ou igual a 10%, e com sintomas da síndrome das pernas inquietas.
ResultadosA mediana de idades dos doentes incluídos foi de 60,0 (intervalo interquartil (IIQ) 10,0) anos; 82,5% eram do sexo masculino. Os valores iniciais medianos da escala de sonolência de Epworth (ESE) e do índice de apneia-hipopneia (IAH) foram de 12,5 (IIQ 9,0) e de 44,1 (IIQ 22,4)/h, respectivamente. A maioria dos doentes (91,3%) estava sob pressão positiva nas vias aéreas em modo automático (auto-adjusting positive airway pressure (APAP)). A insónia prévia ao uso de SVN estava presente em 30% (n=24) dos doentes. A prevalência de IN foi de 21,4% (12/56) e os subtipos de insónia inicial e/ou intermédia foram os mais frequentes (n=11). Foi encontrada uma relação negativa estatisticamente significativa entre a IN e a pressão em 90% do tempo de SVN (P90) (p=0.040).
ConclusõesOs doentes com SAOS sob SVN apresentaram uma prevalência elevada de IN. Os doentes com IN apresentaram níveis inferiores de pressão de SVN comparativamente com os outros.
Insomnia and obstructive sleep apnoea syndrome (OSAS) are the two most common sleep disorders, and both have significant, associated increases in health costs. Although, an earlier epidemiological study reported a prevalence of OSAS of 2–4% in a middle-aged adult population,1 a significantly higher prevalence was found in a more recent study as a result of the different sample features, diagnosis criteria, techniques and epidemiological methodologies applied.2 Similarly, estimates of the prevalence of insomnia depend on the criteria used to define this disorder and more importantly the population studied.3 Insomnia symptoms occur in approximately 33–50% of the adult population; insomnia symptoms with distress or impairment (i.e., general insomnia disorder) in 10–15%; and specific insomnia disorders in 5–10%.3–5 Insomnia, a complex and multifactorial entity, has been explained as a psychological process, with a strong physiological component defined as central nervous system activation or increased arousal activity during sleep.5–8 Some authors suggest that this arousal behaviour may be innate or genetically determined, serving as a substrate upon which various external factors, such as stress, may act to aggravate sleeplessness.9–11 Despite the high prevalence of this symptom and its significant psychosocial consequences, little is known about potential interactions or associations between the two disorders – OSAS and insomnia. Some studies have shown that 40–50% of patients with sleep-disordered breathing present problematic insomnia symptoms before beginning any type of treatment.5,12–16 Chung14 documented a prevalence of 6%, 26% and 19% of initial, intermediate, and final insomnia respectively in patients with OSAS, noting that patients with initial insomnia had significantly lower apnoea–hypopnoea (AHI) and arousal indices. Although insomnia is a common complaint in patients who are evaluated for OSAS, some believe that these two disorders are not strongly associated.17,18 However, other studies suggest that co-morbidity of insomnia in OSAS patients may lead to increased severity of OSAS, decrease in OSAS treatment compliance, and that patients with both conditions may experience more symptoms relating to depression, anxiety, and stress.13,19–21 Given the substantial overlap in symptoms between insomnia and OSAS, evaluation and treatment of these two conditions can be challenging and will require multidisciplinary collaboration among sleep specialists.
Despite the inconclusive and sometimes contradictory results concerning the relationship between insomnia and OSAS before treatment, there is little doubt that these two disorders can coexist ad initium. However, in clinical practice it has been observed that patients with OSAS and no insomnia at baseline develop this symptom after beginning treatment with nocturnal ventilatory support (NVS). In this context, we usually consider this complaint as new-onset insomnia (NOI) or de novo insomnia. This is the justification for our aim to evaluate the prevalence of NOI in patients with OSAS under NVS, and to determine its associated risk factors.
Material and methodsSubjectsWe performed a descriptive cross-sectional study enrolling 80 patients with OSAS, diagnosed by cardiorespiratory poligraphy and/or polysomnography, who were followed in our Sleep Disordered Breathing Clinic. The severity of OSAS was classified as mild (AHI: 5–15/h), moderate (AHI: 15–30/h), and severe (AHI >30/h), according to established criteria.22 All patients were under NVS with auto-adjusting positive airway pressure (APAP), fixed continuous-PAP (CPAP) or bi-level PAP (BiPAP), from a variety of models and ventilator manufacturers (Goodknight® 420 Evolution [Tyco]; S8 AutoSet Spirit® II [ResMed]; REMstar® Auto [Respironics]; iSleep20+® [Breas]; Goodknight® 425 ST [Tyco]).
Exclusion criteria were as follows: patients under anxiolytic and/or antidepressant medication; patients with a weight loss of 10% or greater; and patients with clinical features suggestive of restless legs syndrome (RLS).
Our study was approved by the Ethics Committee of Hospital de São João.
Study designWe analyzed demographic variables (gender and age), baseline and current Epworth Sleepiness Scale (ESS), OSAS severity, previous insomnia and NOI, subtypes of NOI, treatment duration and compliance, the number of previous and current hours of sleep, and the presence of an anxious/depressive personality in the total sample.
Previous insomnia was considered present if the patient answered the baseline question “I feel that I have Insomnia” from the Sleep Disorders Questionnaire (SDQ) (version 1.02, Portuguese), as “almost always” (4) or “always” (5).23
After starting treatment, insomnia was evaluated through the SleepMed Insomnia Index Questionnaire (SMIIQ).24 This is a new diagnostic questionnaire, designed to measure insomnia symptoms rapidly and in a simple standardized way. It has not yet been validated, but has a high degree of reliability, as has been previously shown.24 The SMIIQ consists of 10 questions formulated to address important sleep factors, including sleep latency, performance anxiety, first night effect, frequency of awakening, getting back to sleep, total sleep time, perceived sleep quality, and impact on next day function. NOI was considered present when a patient had a SMIIQ score >20 without evidence of previous insomnia. NOI was classified as initial, intermediate, and final, according to the description of symptoms.
Treatment compliance was assessed through NVS equipment software, evaluating the percentage of total days of usage, average number of hours per night, pressure on 90% nighttime (P90) when applied, and residual AHI.
The SDQ was also used to evaluate the presence of an anxious/depressive personality. A patient was classified as having an anxious/depressive personality if he or she scored 4 or 5 in the questions “My sleep is disturbed by sadness or depression” and “My sleep is disturbed by concerns with life situations”.23
Statistical analysisStatistical analysis was performed using the SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). Data were described as median and interquartile range (IQR) for quantitative variables (non-normally distributed variables) and as counts and proportions for qualitative variables. Proportions were compared using Chi-square test or Fisher's exact test whenever appropriate. For comparison between median values in patients with and without NOI the non-parametric Mann–Whitney and Kruskal–Wallis tests for independent samples were used. A p value <0.05 was considered statistically significant.
ResultsThe median age of patients enrolled was 60.0 (IQR 10.0) years, and 82.5% were male. Median initial AHI was 44.1 (IQR 22.4)/h, reflecting the disease severity (mild: 1.2% (n=1); moderate: 17.5% (n=14); severe: 81.3% (n=65)). Median baseline ESS was 12.5 (IQR 9.0), with minimum and maximum values of 0 and 24, respectively. Most of the patients (91.3%; n=73) were under APAP and the remaining under CPAP (7.5%; n=6) and BiPAP (1.2%; n=1). The median values of P90 and fixed pressure were 10.2 (IQR 3.2) cmH2O and 10.5 (IQR 4.1) cmH2O, respectively. Inspiratory-PAP (IPAP) and expiratory-PAP (EPAP) values of the patient under BiPAP were 18 and 12cmH2O, respectively. The median time period with NVS was 26.0 (IQR 34.0) months.
Insomnia at baseline was present in 30% of patients (n=24), but in 11 of these patients, this symptom disappeared after starting treatment.
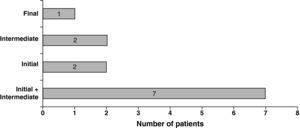
After excluding patients with previous insomnia, we analyzed the remaining 56 patients, divided into two groups according to the presence of NOI. The prevalence of NOI was 21.4% (n=12). Subtypes of NOI are depicted in Fig. 1. The majority of NOI patients were under APAP (n=10), and 2 were under CPAP and BiPAP, respectively.
No significant differences were found between the groups, in relation to gender (p=0.433), age (p=0.826), anxious/depressive personality (p=0.443), OSAS severity (p=0.842), efficacy of treatment measured by residual AHI (p=0.596) or previous and current number of hours of sleep (p>0.05) (Table 1).
Comparison of characteristics between patients with and without new-onset insomnia (NOI).
| Without NOI n=44 | With NOI n=12 | p-Value | |
| Gender, n (%) | |||
| Female | 7 (15.9%) | 3 (25.0%) | 0.433 |
| Male | 37 (84.1%) | 9 (75.0%) | |
| Anxious/depressive personality, n (%) | |||
| No | 35 (79.5%) | 8 (66.7%) | 0.443 |
| Yes | 9 (20.5%) | 4 (33.3%) | |
| Age (years) | 60.0 (9.0) | 61.0 (18.0) | 0.826 |
| SMIIQ score | 8.0 (8.0) | 22.5 (7.0) | <0.001 |
| Initial AHI (events/h) | 44.0 (23.3) | 42.2 (24.8) | 0.842 |
| Residual AHI (events/h) | 2.5 (1.9) | 2.6 (1.1) | 0.596 |
| Previous hours of sleep (h) | 7.0 (1.9) | 7.0 (1.8) | 0.671 |
| Current hours of sleep (h) | 7.0 (2.0) | 6.7 (2.5) | 0.599 |
| Current–previous hours (h) | 0.0 (1.0) | 0.0 (1.9) | 0.749 |
| Treatment | |||
| Duration (months) | 26.0 (36.8) | 22.0 (30.5) | 0.447 |
| Total days of usage (%) | 99.0 (5.4) | 99.0 (24.6) | 0.976 |
| Hours/night (h) | 7.0 (1.5) | 6.5 (2.3) | 0.408 |
| P90 and fixed pressure (cmH2O) | 10.9 (2.8) | 9.2 (3.2) | 0.040 |
Quantitative variables are expressed as median (interquartile range).
AHI, apnoea–hypopnoea index; NOI, new-onset insomnia; P90, pressure on 90% nighttime; SMIIQ, SleepMed Insomnia Index Questionnaire.
The median baseline ESS was lower in NOI patients, but the difference was not statistically significant (with NOI: 11.0 (IQR 11.0) versus without NOI: 12.0 (IQR 6.0); p=0.667). Also, the variation between current and baseline ESS was not statistically significant in either group (with NOI: −5.0 (IQR 7.8) versus without NOI: −6.0 (IQR 7.5); p=0.502) (Table 2).
Baseline Epworth Sleepiness Scale (ESS) and its variation in patients with and without new-onset insomnia (NOI).
| Without NOI n=44 | With NOI n=12 | p-Value | |
| Baseline ESS | 12.0 (6.0) | 11.0 (11.0) | 0.667 |
| Current ESS–baseline ESS | −6.0 (7.5) | −5.0 (7.8) | 0.502 |
Quantitative variables are expressed as median (interquartile range).
ESS: Epworth Sleepiness Scale; NOI: new-onset insomnia.
With regard to treatment compliance, patients with NOI showed a shorter but non-significant duration of treatment (22.0 (IQR 30.5) months versus 26.0 (IQR 36.8) months; p=0.447) and fewer hours of sleep (6.5 (IQR 2.3)h/night versus 7.0 (IQR 1.5)h/night; p=0.408) (Table 1).
A statistically significant negative relation was found between NOI and P90 (p=0.040) (Table 1), although when the different subtypes of NOI were compared by median values of P90, no statistically significant difference was found (p=0.067) (Table 3).
Comparison between pressure on 90% nighttime (P90) and subtypes of new-onset insomnia (NOI).
| Subtype of NOI | n | P90 | p-Value |
| Without NOI | 44 | 10.9 (2.8) | 0.067 |
| Initial NOI | 2 | 6.8 | |
| Intermediate NOI | 2 | 10.0 | |
| Final NOI | 1a | 11.4 | |
| Initial+intermediate NOI | 6b | 9.5 (2.4) |
Quantitative variables are expressed as median (interquartile range).
NOI: new-onset insomnia; P90: pressure on 90% nighttime.
Taking previous studies into account, assessment of insomnia should be included in the management of sleep-disordered breathing before starting treatment, mainly because of its high prevalence and its impact on sleep quality.12–17,19,20 In our OSAS patients, prevalence of previous insomnia was 30%, a slightly lower rate than that described in the literature, which may be the result of the small sample size, a potential limitation of this study. Some authors suggest that further studies on the relationships between insomnia in OSAS patients and NVS are required.12,14,16
We also consider that NOI in OSAS patients deserves evaluation, as we observed that some patients, non-insomniacs at the beginning of treatment, developed symptoms of insomnia after starting NVS. In our population, the prevalence of NOI was 21.4%. In order to eliminate the subjective aspect of this self-reported symptom, we evaluated pre- and post-treatment insomnia through reliable tools, such as SDQ and SMIIQ, respectively.23,24
Patients under anxiolytic and/or antidepressant medication were excluded, as these medications could themselves influence complaints of insomnia, also excluded were those who had a weight loss of 10% or greater, as this could have affected the severity of their sleep-disordered breathing. In addition we excluded patients with RLS, using the diagnostic criteria of the International Restless Legs Syndrome Study Group, as this condition can induce insomnia complaints, like difficulty in falling asleep and maintaining sleep.25,26 In this context, we can add that patients did not present any major medical (digestive, genitourinary, endocrine, musculoskeletal, pulmonary, cardiovascular, etc.) or other sleep disorders (periodic limb movement disorder, circadian rhythm sleep disorders, etc.), and did not have a history of substance abuse, which could have affected the development of insomnia.
The comparative analysis between the two groups, with and without NOI, was based on only 56 patients; we excluded the 24 patients with previous insomnia, as they could have some of the same specific features as NOI patients.
In the general population, insomnia is more common in women and increases with age.3,5,27 In our sample, we did not find a link with gender and age; this may be because there was a predominance of the male gender and homogeneous age in our sample and as stated above, the sample size was small. However, we can develop ideas about different pathogenic mechanisms concerning NOI associated with NVS, like pressure variation related arousals.
Psychological factors and insomnia are interconnected; we know that 40% of insomniacs have a coexisting psychological disorder.3,5,28 Anxiety or depression could affect the development of NOI, but in our sample this relationship was not statistically significant. However, as anxiety and depression were not evaluated through specific questionnaires or by a specialist consultant, these personality features could have been underestimated in our study.
Patients with and without NOI had similar baseline ESS values. Also, the variation between post- and pre-treatment values was similar in both groups. It seems that OSAS symptoms, such as hypersomnia, are not related to insomnia, but to the disease itself, as others have shown.17,22,29
No differences were found concerning treatment compliance, which was good in both groups. However, NOI group presented shorter treatment duration and fewer hours of sleep per night, compared to the other group.
We can speculate about the adaptation to a new treatment during the night as a factor in the worsening quality of sleep. It is possible that NVS itself becomes a cause of frustration and sleep fragmentation, increasing sleep difficulties. This could also explain the predominance of initial and/or intermediate insomnia subtypes, reflecting the difficulty of NOI group in getting to sleep and maintaining its sleep.
In our sample the majority of NOI patients were under APAP (n=10). APAP devices were designed to continuously adjust the applied pressure to the optimal level throughout the night, according to an algorithm, and therefore minimize the mean overnight pressure requirements of patients with OSAS. Because pressure changes occur throughout the sleep period, some authors have suggested that these devices may actually increase sleep fragmentation, which would explain some of the differences in CPAP and APAP performance. However these are not related to the improvement of OSAS and indexes, such as residual AHI, as observed in the population studied.30–32 Despite the fact that the frequency of microarousals and sleep fragmentation induced by APAP devices appears to be small,31 it might be enough to explain the development of insomnia after treatment had started.
We found a statistically significant negative relation between NOI and P90. This finding cannot be explained by the severity of OSAS,22,33 as there was no difference in initial AHI between the two groups. Also, NOI group was treated effectively, as shown by residual AHI, so we cannot explain this relation by unsolved respiratory events. We can speculate that the number of arousals is the cause of the relationship found, but we did not monitor patients with a polysomnography after treatment had begun. In addition, as insomniacs are awake for longer periods than non-insomniacs, they do not have as many events to solve as the other group, and consequently the pressure on the device can be lower. Although the present work was not designed to study patients with previous insomnia or the influence of NVS on this symptom, we found that median value of P90 was higher among those who maintained insomnia (n=13) than in those who resolved it (n=11), although without statistical significance (data not shown). This finding that P90 was higher in insomniac patients may suggest that previous insomnia and NOI have distinct pathogenesis.
As limitations of the study, we draw attention to the small sample size and the uneven number of patients observed in the two groups analyzed. The number of OSAS patients enrolled was limited, because the original intention of our study was primarily exploratory, aimed at confirming a clinical suspicion, the development of insomnia associated with OSAS treatment, something which has not been mentioned so far in the literature. Given the present data, we now believe that this is an important area which requires further research in order to pursue other types of potential predictive factors and etiopathogenic mechanisms. Furthermore, we can speculate about the use of different tools for the evaluation of insomnia before and after initiating NVS. Using the same questionnaire would address the lack of uniformity concerning this issue. The idea of using the SMIIQ was to characterize NOI patients better after they had been identified. However, our “final” sample had only 56 patients, since 24 had previous insomnia and had to be excluded, leaving only 12 with NOI. This is why we think this investigation of NOI features in this context should be analyzed in a larger group of patients. The use of SMIIQ, without its Sleep Matrix refinement,24 could also be challenged in relation to the differential diagnosis between insomnia and other sleep disorders. Yet, we emphasize that our main aim was to evaluate a type of insomnia that was associated with the use of NVS, and not related to OSAS itself. In this context, we only applied the SMIIQ score as a quick and easy way to screen insomnia (>20), and because it is known to have high reliability in insomniac patients; the calculation of the Sleep Matrix was really outside our objectives, and would have made the study more complex to interpret. Furthermore, as we can see in Table 2, the median baseline ESS in NOI patients was not higher than 11, which could indicate the development of NOI independently of OSAS or other sleep disorders.
Another potential limitation of the study is that the time that elapsed, from the beginning of the NVS to the development of complaints of insomnia, is not specified. However the SMIIQ was applied at the 3-month follow-up visit or later, and we can state that those patients did not present previous insomnia, nor did they develop any co-morbid situation (medical disorder or substance abuse) after initiating NVS which could have affected the insomnia.
Although we have stressed the lack of a proper instrument for evaluating personality features, such as anxiety or depression, we think that this has been dealt with the exclusion of patients with previous insomnia, who could have presented a co-morbid anxious personality which was not revealed by the answers to the relevant SDQ questions.
ConclusionsOSAS patients under NVS presented a high prevalence of NOI (21.4%). Gender, age, OSAS severity, treatment compliance, and the presence of an anxious/depressive personality were not associated with the development of NOI. Patients with NOI presented lower levels of pressure using NVS. Longer and larger studies are needed to determine whether NOI exists as an independent “entity” or merely secondary to NVS.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors would like to sincerely thank all Sleep Technicians who manually reviewed all sleep studies.
| 0 | 1 | 2 | 3 | 4 | |
| 1. No geral, considera que tem problemas com o seu sono? | |||||
| 2. É fácil para si adormecer? | |||||
| 3. Sempre que tem preocupações isso traduz-se em problemas no seu sono? | |||||
| 4. Acorda facilmente com ruídos durante a noite? | |||||
| 5. Que nível de incómodo sente quando dorme noutro local/cama diferente do habitual? | |||||
| 6. O seu sono é perturbado com frequentes despertares durante a noite? | |||||
| 7. Adormecer após acordar durante a noite é um problema? | |||||
| 8. Sente-se descansado(a) no dia seguinte, após uma noite de sono? | |||||
| 9. Acha que dorme o número de horas suficientes por noite? | |||||
| 10. Quanto é que a qualidade do seu sono o(a) afecta nas suas actividades diárias (fadiga, humor, irritabilidade)? | |||||
| Total (0–40) |
Escala:
0=Sem problemas com o meu sono.
1=Algum (pouco) problema com o meu sono.
2=Problema moderado com o meu sono.
3=Problema moderado/grave com o meu sono.
4=Problema grave com o meu sono que afecta a minha vida.
Please cite this article as: Caetano Mota, P. Prevalência de insónia de novo em doentes com síndrome de apneia obstrutiva do sono tratados com suporte ventilatório nocturno. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2011.06.016.