To evaluate the prevalence of Sleep Apnea–Hypopnea Syndrome (SAHS) in patients who were admitted with Acute Coronary Syndrome (ACS) to the Coronary Care Unit (CCU) and the clinical predictors of SAHS in patients with ACS and to compare the results of the simple sleep test (SST) with polysomnography (PSG).
MethodsThis was a prospective study that included patients who were admitted to the CCU with ACS, which was confirmed by coronary angiography. Demographic and anthropometric data, cardiovascular risk factors and measures on the Epworth Sleepiness Scale were collected. The SST was conducted with the ApneaLink™ device during hospitalization or after discharge. Patients with an apnea–hypopnea index (AHI) ≥10/h were invited to participate in PSG.
ResultsNinety-one patients with ACS were consecutively included over 4 months. Of the 58 patients who completed the study 43 (74.1%) were male. The mean age was 61.7±12.2 years, and the mean body mass index was 27.4±3.5kg/m2. The median time for SST performance was 17.5 days. This study was compatible with SAHS in 25 cases (43.1%). Patients who had an AHI ≥10/h in the SST were submitted to PSG and SST simultaneously. The median interval between the ACS and the execution of PSG was 30 days. PSG confirmed that all the cases detected by SST were positive.
ConclusionIn our study, we found a high prevalence of SAHS in patients who were admitted to the CCU with ACS (43.1%). These results support the need for SAHS screening in patients who are hospitalized with ACS. The SST may have a role in the screening of SAHS in this population.
Avaliar a prevalência da Síndrome de Apneia-Hipopneia do Sono (SAHS) em doentes internados na Unidade de Cuidados Intensivos Coronários (UCIC); determinar fatores clínicos preditivos de SAHS; comparar os resultados obtidos com o estudo de sono simplificado (ESS) com os da polissonografia (PSG).
MétodosEstudo prospetivo de doentes internados na UCIC com Síndrome Coronária Aguda (SCA), confirmado por coronariografia. Foram avaliados dados demográficos e antropométricos, fatores de risco cardiovascular e valores da escala de sonolência de Epworth. O ESS foi realizado com ApneaLinkTM durante o internamento ou após a alta. Os doentes com índice de apneia-hipopneia (IAH) ≥10/h foram convidados a realizar PSG.
ResultadosDurante 4 meses foram selecionados consecutivamente 91 doentes com SCA. Cinquenta e oito doentes completaram o estudo, sendo 43 (74,1%) do sexo masculino, média etária de 61,7±12,2 anos e índice de massa corporal médio de 27,4±3,5kg/m2. A mediana de tempo para realização do ESS foi de 17,5 dias. O estudo foi compatível com SAHS em 25 casos (43,1%). Aos doentes com IAH ≥10/h no ESS foi proposta a realização de PSG e ESS em simultâneo. A mediana do tempo entre SCA e a PSG foi de 30 dias. A PSG confirmou a positividade de todos os casos detetados pelo ESS.
ConclusãoNo nosso estudo detetámos uma elevada prevalência de SAHS em doentes com SCA internados na UCIC (43,1%). Os resultados suportam a necessidade de um método de rastreio da SAHS em doentes internados com SCA. O ESS pode ter um papel importante no rastreio da SAHS nesta população.
Sleep Apnea–Hypopnea Syndrome (SAHS) is a significant public health problem because of its high prevalence and its association with hypersomnia, motor vehicle accidents, cardiovascular morbidity, cognitive disorders, anxiety, depression and metabolic abnormalities.1,2 Young et al. observed an SAHS prevalence of 2–4% in the adult population.3 However, recent studies indicate that 3.7–26% of the population has an apnea–hypopnea index (AHI) that is greater than 5 events per hour.1 If we consider an AHI >5/h and the presence of hypersomnia to be joint indicators of SAHS, the estimated prevalence of SAHS is 1.2–7.5%.1
Ischemic heart disease, including Acute Coronary Syndrome (ACS), is also a serious problem due to its high prevalence, associated complications and mortality. Several studies have demonstrated an independent association between SAHS and ACS, which suggests that SAHS should be considered a risk factor in patients with ACS.4–6 The changes that are observed in SAHS, particularly the intermittent hypoxemia, acidosis and sympathetic vasoconstriction, may lead to hemodynamic stress, which is particularly important in patients with coronary heart disease and can cause myocardial ischemia or nocturnal angina.6–9
Most patients with SAHS remain undiagnosed and untreated10–12; this is particularly important in patients with cardiovascular disease whose SAHS treatment is associated with a decrease in the occurrence of new cardiovascular events.13
The aim of this study was to evaluate the prevalence of SAHS in patients with ACS, determine the clinical predictive factors of SAHS and compare the correlation of the AHI that is obtained by a simplified sleep study device (SSD) with the AHI that is obtained by polysomnography (PSG).
MethodsSampleThis was a prospective study of 91 consecutive patients who were admitted with ACS to the Coronary Intensive Care Unit (CICU) of our hospital between May and August 2009 with a lesion that was demonstrated by angiography.
The exclusion criteria were as follows: non-resident in the reference area of the hospital, a previous diagnosis of SAHS, confused state, the ingestion of sedatives in the previous 24h, hemodynamic instability, requiring oxygen therapy and patients who refused to participate in the study.
Permission was obtained from the Hospital Ethics Committee in accordance with the Declaration of Helsinki, and signed informed consent was obtained from the patients before the study.
Study designThe following information was collected: demographics, drug habits, sleep habits, comorbidities, medication, symptoms that were suggestive of SAHS, the Epworth Sleepiness Scale (ESS), clinical data and the timing of acute coronary events, physical exam and anthropometric measurements.
Patients who met the inclusion criteria underwent a sleep study using the SSD ApneaLink™ (ResMed Corporation, Poway, California) as the first step. The ApneaLink™ (AL) has two sensors, a flow nasal cannula and oximetry, which provide information on four variables: respiratory flow, snoring, oxygen saturation and heart rate. The data that are collected by AL can be analyzed automatically using dedicated software (version 8) or manually to obtain an AHI and other parameters. The scoring of the collected data by AL was performed automatically using software and manually by the same physician who did not know the results of the automatic scoring.
Apnea was defined as a decrease of 90% of the signal flow for at least 10s. Hypopnea was defined as a reduction in nasal flow ≥30% from baseline for at least 10s that was accompanied by oxygen desaturation ≥4%. Studies with less than 5h of registration were excluded. Validation studies of the AL show that this device has good sensitivity and specificity for AHI ≥10/h in PSG of 82.1% and 83.9%, respectively.12,14,15 Therefore, AL was considered positive when AHI ≥10/h.
Patients with AHI ≥10/h using AL by manual scoring were selected to undergo PSG and AL simultaneously. During the study, the nasal cannula was connected to a “Y” connector, allowing for the simultaneous recording of nasal airflow in the AL and the PSG. A PSG was performed in a sleep laboratory using an Embla® N7000 (Embla Systems, Broomfield, CO, USA), and the following physiological variables were recorded: 6-channel electroencephalography, electrooculography, electromyography at the chin and anterior tibia, electrocardiography, thermistor and nasal cannula, bands for thoracic and abdominal effort (i.e., inductance plethysmography), microphone, body position, oximetry, heart rate and video. The scoring of the PSG was performed manually without the investigator having prior knowledge of the results of the AL or patient information, as recommended by the American Academy of Sleep Medicine (AASM) (2007).16
The SAHS was defined according to the criteria of the International Classification of Sleep Disorders (ICSD-2), which was proposed by AASM.17 SAHS was diagnosed if AHI was between 5 and 14.9/h as measured by PSG and patients had at least one of the symptoms, such as snoring, fatigue, daytime sleepiness and apnea observed during sleep, or if individuals had an AHI ≥15/h regardless of whether they had any additional symptoms.
Statistical analysisAll variables were tested for normal distribution using a frequency histogram and the Kolmogorov–Smirnov test. The difference between two means was determined using Student's t-test or the Welch test. When the variables had a normal distribution, Student's t-test was used, and if the variables did not have a normal distribution, the Welch test was performed. Proportions and categorical variables were analyzed using the Chi-square test or Fisher's exact test when appropriate. Differences were statistically significant at a p value <0.05. A correlation analysis was performed using the Pearson correlation coefficient and the intra-class correlation coefficient. The Bland–Altman plot is a graphical representation of the observed differences between the two techniques (PSG and AL) compared with its average; therefore, results with small differences in the average show little systematic bias. The data were statistically analyzed using the MedCalc® software (version 9.3, Mariakerke, Belgium).
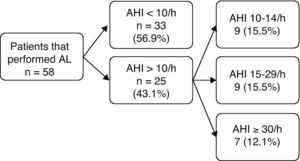
ResultsThe study population consisted of 91 patients with ACS who were admitted to the CICU during a period of 4 months. Thirty-three patients were excluded: 24 did not live in the reference area of the hospital, 7 refused to participate in the study, 1 died a few hours after admission, and 1 patient had a previous diagnosis of SAHS, so 58 patients were included in the final analysis.
Most of the patients were male (74%). The mean age was 61.7 years, and the mean body mass index (BMI) was 27.4kg/m2. The patient characteristics are described in Table 1.
Demographic features, prevalence and comorbidities of the 58 patients that were included in the study.
| Results | |
| Demographic features | |
| Age (years) | 61.7±12.2 (22 – 87) |
| BMI (kg/m2) | 27.4±3.5 (20.8 – 37.5) |
| Sex | |
| Men | 43 (74.1%) |
| Women | 15 (25.9%) |
| AHI prevalence | |
| AHI≥10–14/h | 9 (15.5%) |
| AHI≥15–29/h | 9 (15.5%) |
| AHI≥30/h | 7 (12.1%) |
| Comorbidities | |
| Dyslipidemia | 43 (74.1%) |
| Hypertension | 42 (72.4%) |
| Diabetes Mellitus Type 2 | 17 (29.3%) |
| Previous ischemic heart disease | 13 (22.4%) |
Data are presented in numbers (%), except age and BMI (body mass index), as mean±SD. Apnea–hypopnea index (AHI) values with ApneaLink™.
Twenty-eight patients (48.3%) performed the study with AL during hospitalization, and 30 patients (51.7%) participated after discharge, the median interval between the ACS and the performance of the AL was 4 and 17.5 days, respectively. Based on data from the AL study, 43.1% of the patients had an AHI ≥10/h, 27.6% had an AHI ≥15/h, and 12.1% had an AHI ≥30/h (Table 1 and Fig. 1).
Patients were divided into two groups according to the results of the AL (AHI<10/h versus AHI ≥10/h) and anthropometric data, and clinical features and clinical histories were compared. Statistically significant differences were observed between the two groups in various variables: obesity; neck, waist and hip circumferences; Mallampati classification; history of witnessed apneas and nocturia; and ESS and a history of ischemic heart disease (Table 2). In 48% of the patients with AHI ≥10/h, the cardiac symptoms started during the night (p=0.009), and in most of these patients, the ECG registration did not have a ST elevation (p=0.043).
Clinical and anthropometric features.
| AHI<10/h (n=33) | AHI≥10/h (n=25) | p values | |
| Men | 22 (67%) | 21 (84%) | NS |
| Age (years) | 60±12 | 64±12 | NS |
| Obesity (BMI≥30kg/m2) | 4 (12%) | 9 (36%) | 0.031* |
| Neck circumference (cm) | 34±5 | 39±5 | <0.001** |
| Waist circumference (cm) | 93±10 | 99±9 | 0.012** |
| Hip circumference (cm) | 94±10 | 100±8 | 0.034** |
| Mallampati III+IV | 7 (12%) | 21 (36%) | <0.001* |
| Smoker | 11 (33%) | 7 (28%) | NS |
| Alcohol habits (usual/occasional) | 27 (81%) | 19 (76%) | NS |
| Number of coffees/day | 1.8±1.6 | 1.6±1.4 | NS |
| Number of sleep hours | 6.9±1.7 | 6.8±1.8 | NS |
| Hypertension | 23 (70%) | 19 (76%) | NS |
| Dyslipidemia | 24 (73%) | 19 (76%) | NS |
| Diabetes mellitus | 8 (24%) | 9 (36%) | NS |
| Previous ischemic heart disease | 4 (12%) | 9 (36%) | 0.031* |
| Snoring | 26 (79%) | 21 (84%) | NS |
| Witnessed apneas | 4 (12%) | 12 (48%) | 0.002* |
| Sleep fragmentation | 8 (24%) | 8 (32%) | NS |
| Nocturia (number) | 1±0.9 | 2±0.9 | 0.018** |
| Restless sleep | 7 (21%) | 8 (24%) | NS |
| Non-restorative sleep | 9 (27%) | 9 (36%) | NS |
| Seizures | 3 (9%) | 7 (28%) | NS |
| Memory change | 13 (39%) | 13 (52%) | NS |
| Concentration change | 8 (24%) | 9 (36%) | NS |
| Epworth sleepiness scale | 4.5±2.6 | 6.4±4.1 | 0.042*** |
| Epworth sleepiness scale ≥ 10 | 2 (6%) | 5 (20%) | NS |
| Hours of onset of symptoms of ACS | |||
| 22:01 to 06:00 | 4 (12%) | 12 (48%) | 0.009* |
| 06:01 to 14:00 | 15 (45%) | 6 (24%) | |
| 14:01 to 22:00 | 14 (42%) | 7 (20%) | |
| ACS without ST elevation | 11 (33%) | 15 (69%) | 0.043* |
| Number of affected vessels | 1.7±0.8 | 2.0±0.9 | NS |
| Ejection fraction | 58±14 | 59±14 | NS |
| Interventricular septum hypertrophy | 10 (30%) | 9 (36%) | NS |
Data are presented in number (%) and mean±SD. BMI: body mass index; ACS: Acute Coronary Syndrome; NS: not statistically significant.
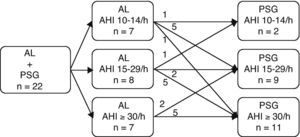
Of the 25 patients with AHI ≥10/h in the AL, 3 refused to undergo the PSG and AL simultaneously. From the ACS, the median time to perform the PSG was 30 days. The PSG confirmed the positivity of all cases of SAHS that were detected by AL. A comparison between the AHI results that were obtained with the first AL and the PSG showed that 14 patients (24.1%) had changed their classification of SAHS after the conclusion of the PSG (Fig. 2). Six patients were classified with mild AL after the conclusion of the PSG, 5 patients were classified as moderate, and 1 patient was severe. Five patients who were initially classified as moderate became severe after PSG. Three patients were classified as less severe after PSG: 1 patient was classified as moderate and after PSG, this patient was categorized as mild; 2 patients who were classified initially as severe were moderate after PSG. Two patients showed predominant central apneas in the PSG.
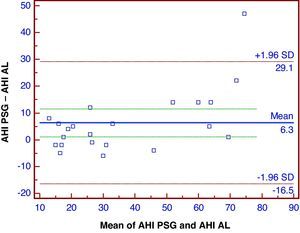
A Bland–Altman plot of the data showed a reasonable distribution of the differences between the AHI from PSG and AL that were performed simultaneously (Fig. 3). Compared to PSG, the AL device undervalued the AHI score by an average of 6.3 events/h, and this underevaluation occurred mainly for AHI values >30/h.
The AL data showed a very good correlation with AHI results from PSG with a Pearson correlation coefficient of 0.91 (0.79–0.96) and an intra-class correlation of 0.93 (0.82–0.97).
According to the definition of the AASM,16 all of the patients who underwent PSG satisfied the criteria for SAHS. As a result, some patients voluntarily started treatment with autoCPAP (19 patients) or Servo-Ventilation (2 patients) as indicated.
DiscussionIn our study, we detected a high prevalence of SAHS (43.1%) in patients who were admitted to a CICU with ACS, and 27.6% were classified as moderate or severe using AL. The PSG confirmed the positivity of all of the cases that were detected by the AL. Our results are consistent with previous studies that describe an association between SAHS and coronary heart disease.4,6,7,18,19 A study performed in another Portuguese hospital found a prevalence of SAHS in 65.9% of patients, 22.7% of which were severe.20
Most of the patients who were admitted in the CICU with ACS were male, with a mean age of 61.7 years and a mean BMI of 27.4kg/m2, which is in contrast to the higher prevalence of obese patients that is observed in SAHS. These patients had a high prevalence of hypertension and dyslipidemia.
About half of these patients performed the SSD with AL during hospitalization (during the acute phase), and the other patients performed the study after discharge at home (during a stable phase). This procedure was performed to avoid possible bias due to medication that is administered on admission, differing degrees of cardiac dysfunction during the acute phase that could lead to central events or a poor quality of sleep that is secondary to stress or to the incessant activity of the intensive care unit.
A comparison of the two groups of patients (AHI<10/h versus AHI≥10/h) showed that a greater number of patients with SAHS were obese, with higher cervical, abdominal and hip circumferences, and more patients were classified as Mallampati III–IV. These are typical features of a patient with SAHS.
The group of patients with SAHS had nocturia in addition to witnessed apneas in greater proportions than patients without SAHS, which are two common symptoms of this disease. Although some patients had witnessed apneas, they had not consulted their physician, which reflects the limited information that is available to the population about this symptom.
It should be noted that, although patients with SAHS had a higher mean value in the ESS than patients without SAHS, this value was lower than the reference value for excessive sleepiness (ESS≥10). Therefore, this value does not seem to be a determining factor in the diagnosis of SAHS in these patients. The limitations of ESS are known, with patients sometimes underestimating their sleepiness, and the existence of patients with severe SAHS without excessive sleepiness is controversial.21
Therefore, higher values in anthropometric data, class III or IV in Mallampati classification, a history of nocturia or witnessed apneas can suggest the presence of SAHS. However, the absence of these data does not preclude SAHS.
The group of patients with SAHS had a higher number of previous episodes of ischemic heart disease (e.g., angina, myocardial infarction), which could be explained by a greater vulnerability of patients with coronary disease to intermittent hypoxemia and sympathetic activation. In patients with SAHS, recurrent apneas and hypopneas are associated with desaturations and arousals that are followed by sympathetic activation, which in turn causes an increase in heart rate and systolic blood pressure. Tachycardia and systolic blood pressure elevation increase myocardial oxygen consumption, which may be compromised in patients with coronary artery disease.4,9,13 Moreover, chronic intermittent hypoxia induces inflammatory mediators, oxidative stress and endothelial dysfunction that lead to atherosclerosis.8,22
Almost half of the patients had their onset of ACS symptoms at night, which is consistent with the results that have been obtained in other studies.8,18,5 In the general population, the onset of ACS is between 6 and 11h, but in patients with SAHS, the onset occurs predominantly between 22 and 6h. This observation suggests that SAHS may precipitate ACS at night by the mechanisms described above and that SCA can contribute to a predisposition for nocturnal sudden cardiac death that is observed in patients with SAHS.9,23,24 However, a relationship between the severity and the extent of the coronary disease (e.g., the number of vessels affected) seen on angiography and the presence of SAHS was not detected. The most frequent electrocardiographic alteration was non-ST elevation, which may be associated with an increase in myocardial oxygen consumption after an apnea, when the oxyhemoglobin saturation is at the lowest, due to an increase in blood pressure and heart rate.7,5 Central respiratory events and brief episodes of Cheyne–Stokes breathing were frequently detected and were significant in 2 patients, although patients had a good ejection fraction on average.
The time between the ACS and PSG was reasonable and avoided some possible bias that could have changed the results, including changes in lifestyle that could lead to weight loss or changes in smoking or alcohol habits.
A comparison of the AHI in the simultaneous PSG and AL showed a good correlation, which confirmed the reliability of this device that has been observed in other studies.12,14,15
Patients with obstructive Sleep Apnea Syndrome (OSAS) were offered therapeutic procedures, such as hygienic-dietetic measures that are consensual in OSAS and CPAP. Because the OSAS treatment in patients with ACS reduced cardiovascular risk, which was defined as cardiovascular death, ACS, hospitalizations for heart failure or the need of coronary revascularization. However, the time to the occurrence of these events is longer.9,13,25–27
The main limitation of this study was the relatively small sample size that was used because of the exclusion of a large number of patients that did not live in Algarve, particularly a large number of tourists that would not have allowed for a follow-up and conclusion in the study.
One enigmatic point is the possibility that the number of respiratory events that were detected initially decreases with time, which has been suggested in a study conducted in Greece28; in this study, the authors concluded that there was a high prevalence of SAHS during the acute phase of ACS (54%), and this prevalence decreased 6 months after ACS and persisted in 21% of the patients, which indicates that this abnormality may be transient. Possible reasons for this decrease in prevalence are the use of certain drugs (e.g., narcotic analgesics and hypnotic/anxiolytic agents) that may affect breathing while asleep, the fact that patients are more likely to lie supine in the CCU and acute cardiovascular pathology which may result in abnormal breathing during sleep with a tendency to central apnea events.6 However, more studies are necessary to confirm this hypothesis.
ConclusionIn summary, our findings show a high prevalence of SAHS in patients with ACS. Considering the high prevalence of SAHS in patients with ACS, it seems justified to include a sleep study in the research protocol of these patients. Although PSG is the gold standard diagnostic test, it is impossible to perform this procedure in all of these patients due to limited health-care resources and the long-term delay for performance. Considering the good correlation between the SSD with ApneaLink™ and PSG and the easy use and low cost of these devices, they could be useful as a first line diagnostic in collaboration with the Sleep outpatient clinic. In the context of ACS, these factors should be investigated and treated with other cardiovascular risk factors, such as hypertension, diabetes, smoking and dyslipidemia, and the diagnosis and treatment of SAHS may be important in the secondary prevention of new cardiovascular events.
Conflicts of interestThe authors have no conflicts of interest to declare.
We thank Linde Homecare and Resmed that lent us the devices for the simplified sleep study.
Please cite this article as: Areias V. Síndrome da Apneia-Hipopneia do Sono e Síndrome Coronária Aguda – Uma Associação a Não Esquecer. Rev Port Pneumol. 2012. doi:10.1016/j.rppneu.2011.07.004.