It is well-established that HIV patients are at high risk of opportunistic infections (OI), like the ones caused by Pneumocystis jirovecii, a worldwide pathogen implicated in interstitial pneumonia (PcP). We present a case of a newly diagnosed HIV-1 patient with multiple OI, including a persistent form of PcP, an invasive aspergillosis (IA), cytomegalovirus and Mycobacterium xenopi lung infection. We describe the combination of laboratorial screening, surgery and antimicrobial therapy which were crucial for patient recovery.
Como é sabido, nos doentes com infeção por vírus da imunodeficiência humana (VIH) existe um alto risco de ocorrência de infeções oportunistas (IO), tais como as infeções por Pneumocystis jirovecii, um agente patogénico com distribuição mundial, que provoca pneumonia intersticial (PPc). Apresentamos um caso de um doente recém-diagnosticado com infeção por VIH-1 e múltiplas IO pulmonares, incluindo uma forma persistente de PPc, aspergilose invasiva (AI), e infeções por citomegalovírus e por Mycobacterium xenopi. Descrevemos a combinação de fatores cruciais para a recuperação do doente, que incluíram a obtenção de dados laboratoriais, intervenção cirúrgica e múltipla terapêutica antimicrobiana.
A 59-year-old previously healthy man presented with a four-month history of progressive weakness, dysphagia, loss of appetite, weigh loss and occasional cough with purulent sputum. In November 2007 he was admitted with acute dyspnea. He was febrile (38.0°C), exhibited an oropharyngeal trush, and had inspiratory crackles in both hemithoraxes. Blood analysis displayed a partial respiratory failure (pO2 66mmHg, pCO2 34mmHg); the chest radiography revealed a marked diffuse bilateral interstitial nodular infiltrate. Search for HIV antibodies (ELISA) was positive. A clinical diagnosis of PcP, oral and esophageal candidiasis were assumed. Cotrimoxazole (1960mg q.i.d.), prednisolone (40mg b.i.d. i.v.) and fluconazole (200mg q.d. i.v.) were started.
Upper gastrointestinal endoscopy confirmed esophageal candidiasis. Fluconazole was stopped after two weeks of treatment, and cotrimoxazole dose was reduced to 960mg q.d., after 21 days. Prednisolone was tapered to 10mg q.d. The remaining analysis presented a peripheral blood TCD4+ of 21cell/μl (5%) and an HIV-1 viral load of 307,285copies/ml.
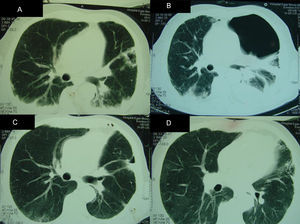
Partial clinical improvement was observed with the above therapy, and the patient remained febrile. At the fourth week of hospitalization, a computed tomography (CT) of the thorax revealed a bilateral alveolar condensation and effusion, and a cavity with a halo sign in the left lung (Fig. 1). A broncoalveolar lavage (BAL 1) was collected during a bronchoscopy. A high burden of P. jirovecii cysts (4–30 cysts in one field at x1000 magnification) was found using indirect immunofluorescence with monoclonal antibodies (IF) (MonoFluo kit P. jirovecii; Bio-Rad, France). The presence of P. jirovecii organisms was confirmed by amplification of the mitochondrial large-subunit rRNA (mtLSU rRNA) gene using nested-PCR. Likewise, Aspergillus fumigatus was revealed in BAL culture. Therapy was modified to clindamycin (600mg q.i.d.), primaquine (30mg q.d.) and voriconazole (200mg b.i.d after a loading dose).
Radiologic findings during hospital admission. Computed tomographies (CT) of the thorax demonstrating: (A) (4th week after admission) bilateral pleural effusion and condensation, with an aspergilloma on left hemithorax; (B) (9th week after admission) hydropneumothorax; (C and D) (6th month after admission) improvement of pleural effusion and hydropneumothorax. Aspergilloma was removed during the surgery.
As the patient was febrile, we also searched for pp6 cytomegalovirus (CMV) antigenaemia that was positive (248 infected cells/50,000 leucocytes). Fundoscopy, cerebrospinal fluid analysis, upper and lower gastrointestinal endoscopy did not suggest CMV disease. However, DNA of CMV was found in PCR of BAL 1. Ganciclovir (325mg b.i.d.) was started.
The patient finished the second anti-Pneumocystis treatment (26 days of clindamycin and primaquine) and ganciclovir (after 21 days). Prophylaxis with atovaquone (1500mg q.d.), pyrimethamine (50mg q.d.), and valganciclovir (900mg q.d.) was begun, and voriconazol was maintained.
Eight weeks after admission, 57 days of anti-Pneumocystis treatment and 30 days of voriconazole, were provided. A new BAL fluid was collected (BAL 2), revealing the persistence of a high parasite burden of P. jirovecii (4–30 cysts in one field at x1000 magnification) and A. fumigatus. CMV DNA was still positive in BAL 2. As the patient was apyretic and CMV antigenaemia became negative, we did not reinstitute anti-CMV therapy in full dose. However, the patient maintained significant cough and dyspnea. A new CT of the thorax still revealed alveolar condensation and the aspergilloma cavity with the previous dimensions. We opted to start a third course of PcP therapy (clindamycin and primaquine); and to add caspofungin (50mg q.d. after a loading dose) to voriconazol. Meanwhile, the laboratory noticed the growth of Mycobacterium xenopi in BAL 1. Ethambutol (1200mg q.d.), azithromycin (500mg, q.d.) and levofloxacin (500mg, q.d.) were started. Given the patient's profound immunosuppression, antiretroviral (ARV) therapy was also begun. After excluding HLA-B57 01, and to avoid interactions, we choose abacavir (ABC, 600mg q.d.), lamivudine (3TC, 300mg q.d.) and indinavir (IDV, 800mg t.i.d.).
The two BALs collected during the episode were genetically characterized at five distinct P. jirovecii loci encoding the mitochondrial large-subunit rRNA (mtLSU rRNA), the cytochrome b (CYB), the superoxide dismutase (SOD), the dihydrofolate reductase (DHFR), and the dihydropteroate synthase (DHPS) using PCR with DNA sequencing analysis. Both samples revealed identical multiple genotypes combinations (Table 1).
Pneumocystis jirovecii genotypes detected in the samples analyzed.
| Sample ID | Locus | ||||
| mtLSU rRNA | CYB | SOD | DHFR | DHPS | |
| BAL 1 | Genotype 2 | CYB 1/2a | SOD 1 | DHFR Wt | DHPS Wt |
| BAL 2 | Genotype 2 | CYB 1/2a | SOD 1 | DHFR Wt | DHPS Wt |
| Aspergilloma | Genotype 3 | CYB 1/2a | SOD 1 | DHFR Wt | DHPS 75 |
| Adjacent Pleura | Genotype 1/3a | CYB 1/2a | SOD 1 | DHFR Wt | DHPS 75/78a |
Genotypes nomenclature based on Esteves et al. 2012.12
Wt: wildtype.
One week later, the patient developed a left hydropneumothorax. He was submitted to thoracotomy, pleural decortication and aspergilloma removing (Fig. 1). In the removed tissue, P. jirovecii and A. fumigatus were identified. Two mutations were detected at codons 75 and 78 of the DHPS gene for the first time (GenBank: accession numbers GU479992, GU479993), in which a threonine was present instead of an isoleucine and a proline was present instead of a leucine, respectively. P. jirovecii genotyping results in the pleura tissue and in the aspergilloma sections are summarized in Table 1.
Since surgery, the patient has had a gradual and sustained improvement. He was discharged 16 weeks after admission. Overall, he completed 77 days of PcP therapy (21 days of cotrimoxazole and 56 days course of clindamycin and primaquine); 22 days of ganciclovir; 9 months of voriconazole (with 71 days of caspofungin); and 15 months of anti-bacillary treatment. ARV was later simplified, with indinavir substituted by efavirenze. Twenty months after discharge, the patient regained his lifestyle, had undetectable HIV-1 viral load and displayed 350 TCD4+ lymphocytes/μl (11%).
DiscussionPcP remains a frequent AIDS-defining opportunistic infection (OI) in Europe and in the United States.1 It is considered a complex pathophysiological phenomenon that may involve other fungal, bacterial or viral coinfections. However, few publications described coinfections involving P. jirovecii, Aspergillus, mycobacteria or CMV. Globally, coinfections in PcP are related to a worst outcome of PcP.2–5
Invasive Aspergillosis (IA) is a rare OI in HIV patients, being more frequently identified in individuals with cancer and neutropenia. The subacute presentation of IA presented here is called Chronic Necrotizing Aspergillosis. It characteristically appears in patients moderately immunossupressed (i.e. diabetes and/or low-dose corticosteroids), with underlying structural lung disease.6
Two of the more important factors in Aspergillus pathogenesis are based on the dysfunction of host lung epithelia (a physical and biochemical immune barrier to Aspergillus), and the presence of sialic acid residues in conidia surface, that will bind with high affinity to fibrinogen, to effectively attach to the epithelium.8 In PcP, damaging of the alveolar epithelium, accumulation of surfactant-like material, fibrinogen expression and fibrin deposition are characteristic.7 The combination of HIV infection, corticosteroid therapy and lung epithelial damage produced by P. jirovecii, may have contributed to the development of IA. In fact, the association of these two diseases in HIV-patients was previously suggested in other case reports and in a small retrospective study.3
Risk factors for treatment or prophylaxis failure in PcP are TCD4+ less than 50cell/μl, prior PcP episode and use a cotrimoxazole-sparing regimen.9 Several polymorphisms in P. jirovecii genetic loci are potentially linked with antimicrobial failure, specifically the previously recognized mutations at DHPS codons 55 and 57, associated with sulpha resistance. In our case, those mutations were not noticed; however, new point mutations at codons 75 and 78 were found. Persistence of P. jirovecii after cotrimoxazole therapy suggests, but does not prove, that the change in these two residues may have, somehow, affected DHPS enzymatic activity.10,11 Further studies are required to investigate this relationship.
A substitution of a phenylalanine by a leucine at CYB codon 280 was also detected. CYB mutations are associated with atovaquone exposure; and it has been suggested that point mutations found in the Qo region (one of two coenzyme Q binding sites on cytochrome b) may be related with atovaquone prophylaxis failure. Since the amino acid 280 is not involved in Coenzyme Q or atovaquone binding, this mutation is probably due to genotype variation, and not related to failure of prophylaxis with atovaquone.12,13
Specific P. jirovecii genotypes may be associated with distinctive outcomes of PcP episodes. In previous studies, high parasite burden was more frequently detected in samples with mtLSU rRNA genotype 2/3, SOD 1 and DHFR wildtype; and samples with mtLSU rRNA genotype 2/3 and SOD 1 were more common in patients with the poorest outcome.14,15 The genotypes found in this case may suggest a PcP with more virulent variants (mtLSU rRNA genotype 2/3, SOD 1 and DHFR wildtype).
After nine weeks of anti-Pneumocystis therapy, we detected a high load of P. jirovecii cysts inside the aspergilloma. Local pH, temperature and surrounding fibrosis in aspergilloma cavity may have affected antibiotic penetration, possibly leading to the emergence of resistant mutations. Factors produced by A. fumigatus such as gliotoxin, a potent local immunosuppressor, and elastases, that break the surrounding tissues,8 may have also contributed to the adherence and persistence of P. jirovecii. Although we observed a clinical response to voriconazole, surgery was more strikingly associated with clinical improvement, maybe by diminishing the burden of infection, and perhaps, by eradicating a biological sanctuary for P. jirovecii and Aspergillus multiplication and cooperation.
The clinical significance and pathogenicity of M. xenopi and CMV in the lungs are still controversial. The American Thoracic Society (ATS) states that Mycobacterium xenopi can act as a pathogen, especially in people with lung alterations. It presents more often as a pulmonary cavity, but in HIV patients, disseminated disease is also common. This agent is sometimes implied as a colonizer, or as a water-associated contaminator of medical devices and laboratory equipment, being responsible for “pseudo” outbreaks. ATS criteria for atypical mycobacterial imply exclusion of other causes, pulmonary symptoms and a reliable microbiological finding, like the growth in BAL culture.16 We believed that our patient had significant risk factors and fulfilled criteria for infection. Interestingly, a case of a M. xenopi and Aspergillus pulmonary infection, in a HIV-positive patient with a previous P. jirovecii infection was already previously reported.3 The clinical significance of CMV detection in respiratory specimens remains questionable in HIV patients, compared with transplanted patients where its role is much more established.17 Important data regarding this issue come from autopsy studies. In 75 subjects that died of HIV infection, half of them from respiratory failure, 44 patients (59%) had histologic evidence of CMV infection. In over half of these patients, the CMV was thought to have caused little or no pulmonary dysfunction, but in contrast, CMV was thought to have had some role in 21 of the 44 patients.18 CMV is often identified in the lungs of patients with severe pneumonia, and in HIV setting, especially with PcP. In 111 patients with AIDS and a first episode of PCP, 48% had CMV demonstrated on BAL. There was no difference between CMV positive and negative patients in terms of initial presentation, long-term survival, hospital mortality, or length of hospital stay.19
While the role of these two microorganisms in this setting, and the best approach is still debatable, we opted to start anti-mycobaterial and anti-viral therapy, given his poor evolution and symptoms.
In conclusion, we present a case of a newly diagnosed HIV-1 patient suffering from a persistent PcP, IA and probable lung infections caused by M. xenopi and CMV. We detected genotype profiles associated with more virulent P. jirovecii, and two new point mutations in P. jirovecii DHPS gene were described for the first time. Genetic variation of P. jirovecii in the same host and in different parts of the lung tissue confirmed that this infection could be polyclonal. Despite the current availability of multiple antibacterial, antifungal and antiviral drugs, we wish to signal this combination of factors that can challenge the management of HIV-associated OI.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
These two authors contributed equally to the present work.
Please cite this article as: Ponces Bento D, et al. Coexistência de infecções oportunistas pulmonares num doente com infecção por VIH e uma forma persistente de pneumonia por Pneumocystis jirovecii: caso clínico. Rev Port Pneumol. 2013. http://dx.doi.org/10.1016/j.rppneu.2013.01.005.