On March 11th, the World Health Organization (WHO) declared the novel coronavirus disease (COVID-19) a pandemic.1 Paediatric patients with COVID-19 are underrepresented in this epidemic so that management strategies and standards of care are poorly defined especially in patients with chronic respiratory diseases. Technologically dependent children on a ventilator represent a subgroup with medical complexity that accounts for about 1/3 of economic health resources.2,3 It is important to remember that these patients are mainly affected by chronic "respiratory pump failure” conditions which can aggravate their clinical condition in cases of respiratory tract infections.4 In most of the cases, children are ventilated, either using non-invasive ventilation (NIV), with an single-limb intentional leak circuit, where typically the leak is within the interface, or a single-limb non-intentional leak circuit with an active exhalation valve inserted into the mask. For tracheostomy ventilation (TIPPV) a single-limb non-intentional leak circuit with an active exhalation valve is normally used, however, some patients are managed with single-limb intentional leak circuit or a double limb circuit.5
Paediatric ventilator-dependent patients are reluctant to change their ventilation modes, settings and interface.6,7
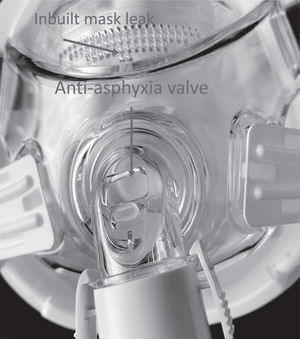
However, during this pandemic, when the patients are admitted to the hospital for an exacerbation of their underlying disease, or with suspected, or positive infection for SARS-COV2 several of the healthcare providers and parents may be in close contact with the children. Where precautions are not taken the risk of transmission of virus due to the ventilator being an aerosol generating procedure (AGP) is high. This is because, expired air aerosolization from ventilators and interfaces8 as well as from plain tracheostomy tube9 may be the cause of contamination of environment and infection in health care workers. It has been demonstrated, in an adult population, that physiotherapy and NIV delivered with vented mask generated large droplets close to the patient but, the fall out of these decreases significantly at 1 m from the patient. NIV using non vented mask and filtered exhalate can reduce the amount of aerosolization.8 In addition, full face or total face masks have a built in “anti-asphyxia valve” that opens when the ventilator does not work properly. The exhaled air from this “anti-asphyxia valve” is also bound to contaminate the environment with the virus.10 Although hospitalization of these patients needs maximum attention in terms of personal protection equipment (PPE) for health care workers and parents the ventilators as well interfaces should be also upgraded to reduce aerosolization of exhaled air see Figs. 2, 4 to 6.
However changing to a non-vented mask has the potential to cause problems. One potential issue is leaving out the expiratory intentional leak. Training and education need to occur in the hospital to prevent adverse events. This can be done by showing a picture of the circuit above the bed. This can also include a reminder to change the heat and moisture exchanger (HME) daily otherwise this will lose the filtration properties required. Another potential risk is a waterlogged filter. This can affect triggering and cause problems with the alarms. These filters need to be checked regularly. Changing from a nasal mask also has the potential to increase the risk of abdominal distention. This can be alleviated by venting the gastrostomy or the feeding tube. Likewise enteral feeding may need to be commenced if ventilator time has increased. The insertion of a nasogastric tube may cause mask leak and this needs to be minimized.
We therefore provide practical recommendations for how to optimize home ventilators circuits and interface configuration in different case scenarios to avoid airborne spread and cross infection, in hospital admissions.
Recommendations for children using a “vented” nasal, full face or total face maskBox 1
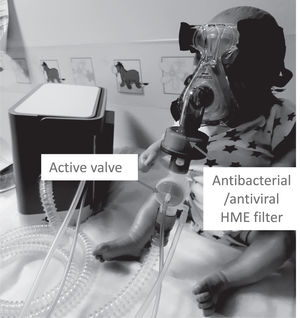
1) Switch to a non-vented mask without an a “anti-asphyxia valve”, (Fig. 1)
2) Attach a antibacterial/antiviral low resistance heat and moisture exchanger (HME)(*) into the mask or after the catheter (for tracheostomy ventilation), (Fig. 2)
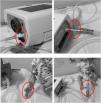
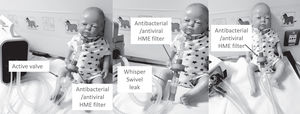
Shows suggestions for switching from a vented mask to a non-vented mask attached to an antibacterial/antiviral heat moisture exchanger (HME) filter, then attached to a controlled leak (Whisper swivel II (Philips, Murraysville, USA). Left picture shows a child mannequin wearing a non–vented nasal mask. Centre picture a full face mask and right picture a total face mask. Note the position of the HME and Whisper Swivel controlled leak (labelled and circled).
3) Next attach an intentional leak for example a whisper swivel II (Phillips Respironics, Murrysville, PA, USA), (Fig. 2)
4) Ensure any wet humidification is removed.
5) Evaluate for an increase in resistance by checking for a possible reduction in the estimated tidal volume when using a time or flow cycled pressure controlled mode without any tidal volume or alveolar ventilation target mode.8 Or in volume controlled mode check for an increase in maximal peak pressure. Perform a measurement of CO2 to assess carbon dioxide level (**)
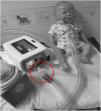
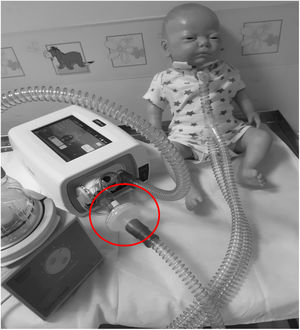
6) For patients who require oxygen, entrain oxygen at the back of the ventilator (Fig. 3). Or insert oxygen before the controlled leak or active valve with a dedicated connector. (Fig. 3)
Top left hand photograph demonstrated that oxygen should be entrained using a dedicated connector where possible (circled). If this is not possible the top right hand photograph illustrates the position of an oxygen connector attached to the ventilator tubing (circled). It can also be placed on, before intentional leak (bottom left photograph) or before the “active valve”, bottom right photograph.
Box 2
1) Remove an a “anti-asphyxia valve” if present
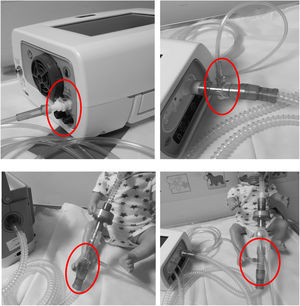
2) Attach the low resistance antibacterial/antiviral HME (*) before the expiratory valve (Fig. 4)
3) Ensure wet humidification is removed
4) Evaluate for an increase in resistance by checking for a possible reduction in the estimated tidal volume when using a time or flow cycled pressure controlled mode without any tidal volume or alveolar ventilation target mode.8 Or in volume controlled mode check for an increase in maximal peak pressure.
5) Perform a measurement of CO2 to assess carbon dioxide level (**)
6) For patients who require oxygen, entrain oxygen at the back of the ventilator (Fig. 3). Or insert oxygen before the controlled leak or active valve with a dedicated connector. (Fig. 3)
Box 3
1) Where possible replace a cuff-less tracheostomy tube to a cuffed tube
2) Remove heated humidification
3) Insert a low resistance antibacterial/antiviral HME (*) between the catheter mount and the circuit (see Fig. 5 for circuit set up)
4) Evaluate for an increase in resistance by checking for a possible reduction in the estimated tidal volume when using a time or flow cycled pressure controlled mode without any tidal volume or alveolar ventilation target mode.8 Or in volume controlled mode check for an increase in maximal peak pressure.
5) Take measurement of CO2 to assess carbon dioxide level (**)
6) In patients who require oxygen, entrain oxygen at the back of the ventilator (Fig. 3). Or insert oxygen before the active valve with a dedicated connector. (Fig. 3)
(*)The addition of a HME will increase the dead space of the circuit. This can cause a drop in the delivered IPAP. If this occurs increasing inspiratory pressure by 1−2 cmH2O solves the problem. The HME will also cause a small increase in the resistance to the circuit. Changing the device triggers sensitivity to be more sensitive which will help to overcome this problem. If triggering remains a problem, switching from a pressure support mode to pressure control with an appropriate back up rate (near to patient’s rate)
(**) We recommend in the first instance continuous transcutaneous CO2 (TcCO2) with pulse oximetry monitoring for the first 24 h to ensure optimal ventilation and no adverse increase in PaCO2 levels as a result of the change in the circuit. If TcCO2 monitoring is unavailable, then this should be replaced with end tidal CO2 (EtCO2) with pulse oximetry monitoring. Where non-invasive monitoring is unavailable percutaneous capillary measurements should be carried out 3 h after changing the mask. If the CO2 levels are satisfactory we then recommend repeating the following morning unless the patient’s SpO2 deteriorates.
Additional scenariosSecretion retentionIf secretions are a problem remove the HME and insert heated humidification with a double limb circuit (see Fig. 6). The expiratory limb should be protected by a hydrophobic antibacterial (HEPA) filter.
It should always remembered when ventilation is required:
- •
Mask on
- •
Ventilator on
- •
Check for nonintentional leaks
- •
Add oxygen if required.
- •
Stop ventilation, oxygen off, ventilator off, mask off
Children receiving any respiratory support should be cared for in airborne isolation rooms at negative pressure whenever possible.
Non-vented face or total face masks should be used as the first choice. A non-vented nasal mask11 should be used as the second choice when the children cannot tolerate the oro-nasal mask or for inability to ventilate the children. Use of pacifiers should be stimulated and mouth-piece ventilation should be avoided.
Staff should adopt full contact, droplet, and airborne isolation precautions whether the patient is positive or suspected of having a COVID-19 infection.
In particular, appropriate type of masks/filtering facepiece respirators (FFR) should be selected according to risk of aerosolization.12–14 If present (as in case of NIV), healthcare personnel should always wear FFR ensuring both droplets and airborne protection (e.g. FFP2, KN95, N95, FFP3).12
Providers and parents should be aware that theoretically, coronaviruses can remain infectious on inanimate surfaces like metal, glass or plastic for up to 9 days.14 Surfaces and equipment like ventilators used for SARS-2- CoV-infected children should be carefully disinfected with appropriate solutions (ie about 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite within 1 min of cleaning).15
Finally, regardless of their respiratory support patients who are admitted to hospital from home with suspicion of having COVID-19–associated respiratory failure, should be closely monitored for deterioration. An emergency care pathway including an escalation plan and ceiling of care should be discussed and documented on arrival.