Swallowing dysfunction has become an increasingly important issue for the quality of life of individuals, including those with interstitial lung disease (ILD). We previously reported two cases of idiopathic pleuroparenchymal fibroelastosis (PPFE) with vocal cord palsy (VCP) and dysphagia,1,2 which may have been caused by recurrent nerve palsy (RNP) due to architectural distortion of the lung. To date, little is known about the role of VCP or RNP in ILD; thus, this study aimed to clarify the clinical and radiological features of ILD with VCP.
Using an electronic medical record search system for registered disease names, we searched for “interstitial pneumonia” or “pulmonary fibrosis” and “vocal cord palsy” or “recurrent nerve palsy” or “hoarseness” (Supplemental Figure). Patients with a history of visits to our hospital between November 28, 2016, and March 31, 2023, were included. We retrospectively identified and evaluated cases of ILD associated with VCP based on medical records. This study was approved by the review board of National Hospital Organization Kinki-Chuo Chest Medical Center (approved number: Rin-2022–120) and complied with the principles of the Declaration of Helsinki.
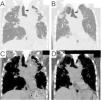
Among 4299 cases of “interstitial pneumonia” or “pulmonary fibrosis,” 20 cases included “vocal cord palsy,” “recurrent nerve palsy,” or “hoarseness” (Supplemental Figure). After excluding the involvement of lung cancer, post-operation, no interstitial lung disease (inappropriate disease name), and insufficient data, four cases were finally identified. In addition to two previously reported cases of idiopathic PPFE,1,2 one case of idiopathic PPFE with usual interstitial pneumonia (case #1; Table 1) and one case of PPFE secondary to fibrotic hypersensitivity pneumonitis (fHP) (case #2; Table 1) were newly identified. The following findings were also common to the previous two cases: Left-sided VCP, radiological PPFE,3 particularly rightward deviation of the trachea caused by fibrosis and contraction of the upper lobe (Fig. 1), and complication of pneumonia.
Cases of interstitial lung disease with vocal cord palsy.
| Case | 1 | 2 |
|---|---|---|
| At ILD diagnosis | ||
| Dx of ILD | Idiopathic PPFE with UIP | PPFE secondary to Fibrotic HP |
| Age, years | 56 | 18 |
| Sex | Female | Female |
| Body mass index, kg/m2 | 14.1 | 15.3 |
| Initial symptoms | Dyspnea, hoarseness | Exertional dyspnea |
| Past history | Nothing particular | Nothing particular |
| Smoking history, pack-years | 0 | 2 |
| Serum biomarkers | ||
| KL-6, U/mL | 332 | 1970 |
| SP-D, ng/mL | 60.5 | 204 |
| Vocal cord palsy | ||
| Side | Left | Left |
| Time to VCP onsetfrom ILD Dx | 5 y | 18 y and 8 m |
| Chest CT findings | ||
| Consolidation with bronchiectasis | (+) | (+) |
| Volume loss of upper lobes | (+) | (+) |
| Upward shift of hilar structures | Bilateral (left-side dominant) | Bilateral (right-side dominant) |
| Trachea deviation | Toward the right side | Toward the right side |
| Flat chest index* | 0.55 | 0.39 |
| Deep suprasternal notch | (-) | (-) |
| PA dilatationMain PA diameter, mm(mPA-D / aAo-D§) | (+) | (+) |
| 35.0(1.04) | 36.2(1.2) | |
| TRPG on UCG, mmHg | 33.2 | 65.9 |
| Pulmonary function tests | ||
| FVC%predicted | 31# | 34.2 |
| FEV1 %predicted | 34.1# | 39.6 |
| FEV1/FVC,% | 92.59# | 98.06 |
| RV/TLC%predicted | 176.5# | 156 |
| Clinical course | ||
| Complication/comorbidity | Pneumonia (three times), acute exacerbation of ILD, left-sided pneumothorax, heart failure | Video-assisted thoracoscopic surgery for right-sided pneumothorax (four times), and left-sided pneumothorax (twice), pneumonia |
| Treatment for ILD | Nintedanib,prednisolone | Prednisolone |
| Prognosis | ||
| Dx of ILD to last follow-up (to lung transplantation) | 10 y and 6 m(7 y and 10 m) | 20 y(19 y and 7 m) |
| Outcome of the last follow-up, Dead/Alive | Alive | Alive |
Ao, Aorta; Dx, Diagnosis; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; HP, hypersensitive pneumonia; ILD, interstitial lung disease; KL-6, Krebs von den Lungen-6; m, months; N.A., not available; PA, pulmonary artery; PPFE, pleuroparenchymal fibroelastosis; RV, residual volume; SP-D, surfactant protein-D; TLC, total lung capacity; TRPG, tricuspid regurgitation pressure gradient; UCG, ultrasound cardiography; VCP, vocal cord palsy; y, year(s).
Flat chest index was defined as the anterior-posterior thoracic dimension on a chest CT scan at the level of the sixth thoracic vertebra, based on the anteroposterior diameter of the thoracic cage/transverse diameter of the thoracic cage ratio, as described previously.6
The main pulmonary artery and ascending aorta were assessed on a transverse image at the level of the pulmonary artery bifurcation. Pulmonary artery enlargement is defined by a ratio of the diameter of the main pulmonary artery (mPA-D) to the diameter of the ascending aorta (aAo-D) [mPA-D / aAo-D] of >1.7
Chest computed tomography scan at the onset of vocal cord palsy. Coronal view of the tracheal bifurcation level. Pulmonary (A) and mediastinal (C) windows in Case #1 and Case #2 (B, D). Rightward deviation of the trachea (black arrows) caused by fibrosis and contraction of the upper lobe is shown (A, B). The aortic arch and pulmonary artery are in close contact (white arrows) with the tracheobronchial and mediastinal deviations (C, D).
Vocal cord palsy was detected only in ILDs with upper-lobe PPFE among various ILDs by the search at our hospital. Little has been reported on patients with ILD with VCP, except for PPFE cases.2 Taken together, upper-lobe PPFE could be a common factor in the development of VCP in ILD.
Several mechanisms underlying VCP have been discussed in PPFE,1,2 and the following structural mechanisms have been postulated to cause RNP in ILD with fibrosis and contraction of the upper lobe:1) traction of the recurrent laryngeal nerve due to tracheobronchial and mediastinal deviation; 2) compression of the left recurrent laryngeal nerve around the aortic window by tracheobronchial and mediastinal deviation and/or pulmonary artery (PA) enlargement resulting from pulmonary hypertension (PH); and 3) stretching of the recurrent laryngeal nerve due to adhesion to the pleura. As an additional contributing factor, fatty tissue depletion in the mediastinal region attributable to emaciation can potentially induce elevated pressure on the recurrent laryngeal nerve.
A rightward deviation of the trachea by contraction of the upper lobe was observed (Fig. 1) because the trachea could easily deviate to the right side due to the presence of the aorta and other structures on the left side of the mediastinum. The palsy manifested on the left side opposite the tracheal deviation toward the right, thereby indicating the first and/or second mechanism above. In addition, in cases #1 and #2, PA enlargement secondary to PH could have affected the palsy by further compressing the nerve around the aortic window. To the best of our knowledge, there has only been one report of VCP associated with secondary PH,4 although Ortner syndrome is known to be caused by PA enlargement due to primary PH or aortic arch aneurysms. Secondary PH is usually milder than primary PH and therefore has less impact on VCP; secondary PH alone is not likely to cause VCP.
A high percentage of the predicted values of the ratio of residual volume to total lung capacity (RV/TLC%predicted) was observed in common with the previous case.1 The data met the diagnostic criteria of PPFE (≥ 115 %),3 and thus, can be largely explained by unique PPFE features due to impaired distension of the chest cage at inspiration. Similarly, low Body mass index is also attributable to the physical characteristics of PPFE. The data met the diagnostic criteria of PPFE (≤ 20 kg/m2),3 which is consistent with the previous cases.1,2 Body mass index and RV/TLC are unique clinical features of PPFE and may help to exclude patients with other fibrosing interstitial pneumonias.3
The significance of our study is that the existence of a definite VCP may suggest more latent ILD cases with paresis or dysfunction of recurrent laryngeal nerve. Recognition of the findings shown herein would lead to a further understanding of dysphagia in ILD. Furthermore, PPFE has been recognized as a complex entity, which is divisible into particular clinical profiles based on radiological findings,5 and the evidence of PPFE with VCP might provide new insight into the PPFE entity.
This study had several limitations. First, the frequency of cases with hoarseness, VCP, and RNP may have been underestimated because this was based on the search for registered disease names. Second, we could not predict the occurrence of VCP in ILDs with tracheobronchial tree and mediastinal deviations, because of the few cases in the retrospective study at one institute and the lack of comparable controls.
In conclusion, VCP was observed only in ILDs with upper-lobe PPFE. PPFE is considered a pivotal factor in the development of VCP in ILD.
Funding sourceNone.
Ethical approvalPatient's data were anonymized.
This study was approved by the review board of National Hospital Organization Kinki-Chuo Chest Medical Center (approved number: Rin-2022-120).
Patient consentThe patients provided informed consent to publish this case report and accompanying images.
Supplemental Figure Patient flow chart of the study.
CRediT authorship contribution statementT. Takimoto: Conceptualization, Resources, Writing – original draft. N. Takeuchi: Resources, Writing – review & editing. Y. Inoue: Resources, Writing – review & editing. T. Arai: Resources, Writing – review & editing.
Y. Inoue reports advisory board participation and lecture fees from Boehringer Ingelheim, Inc., lecture fees from Shionogi & Co Ltd, Kyorin Pharmaceutical Co Ltd, GSK and Novartis, and advisory board participation for Roche/Promedior, Galapagos, Taiho pharma, CSL Behring and Vicore Pharma, outside the submitted work.
NT has received lecture fees from Shionogi & Co., Ltd.
TA has received lecture fees from Boehringer Ingelheim and Shionogi & Co., Ltd.
TT has received lecture fees from Shionogi & Co., Ltd.