The analysis of outcomes from patients with severe asthma treated with omalizumab, using real-life prospective data, should contribute to future informed decisions about this treatment in Portugal.
The aim of this study was to assess the clinical effect of omalizumab in Portuguese patients with severe persistent allergic asthma, considering specifically asthma control and exacerbations.
This was an observational, prospective, multicentre study. Data were collected at routine care over a 12-month period. Disease control was defined by Control of Allergic Rhinitis and Asthma Test (CARAT) global score >24.
All asthma patients already under treatment with omalizumab in 7 departments from 6 Portuguese hospitals were included (n = 48). Most (77%) patients were female and the mean (SD) age was 51.9 (10.2) years old. During the study period, asthma was controlled in 34% of the visits and the 12-month exacerbation rate was 1.7 per patient (0.6 with unscheduled medical care). One-third of the patients needed unscheduled medical care because of asthma and 29% had to start or increase oral corticosteroid. There was still a 41% reduction in the total sum of oral corticosteroids usage from the first to the last trimester of the study.
During routine treatment with omalizumab, Portuguese patients with severe asthma achieved asthma control in 1/3 of the visits and only 1/3 needed unscheduled or Emergency Room care because of asthma exacerbations. These outcomes support the maintenance of the clinical effect during treatment with omalizumab in routine care in Portugal.
Severe asthma was recently defined by the European Respiratory Society/American Thoracic Society as “asthma which requires treatment with high dose inhaled corticosteroids plus a second controller (and/or systemic corticosteroids) to prevent it from becoming ‘uncontrolled’ or asthma which remains ‘uncontrolled’ despite this therapy”.1 It is estimated that up to 15% of asthma patients may have severe asthma.2 These few patients account for a disproportionate part of the burden and healthcare costs associated with asthma.3
Omalizumab is a humanized anti-IgE antibody recommended for treating severe allergic IgE-mediated persistent asthma as an add-on to optimized standard therapy in people aged 6 years or older who need continuous or frequent treatment with oral corticosteroids (OCS) (defined as 4 or more courses in the previous year).4 Previous studies in adults have shown that treatment with omalizumab is associated with an improvement in the control of asthma symptoms, with decreased frequency and use of rescue medication and OCS, and with a reduction in the total asthma-related emergency visits (including hospital admissions, emergency department visits and unscheduled visits to the doctor).4 However, most of these data come from clinical trials, primarily designed to assess treatment efficacy in highly selected patients; this poses problems when generalizing to daily clinical practice. Real-life prospective studies, presented as a pragmatic approach to everyday clinical practice, are fundamental to assess the impact of omalizumab treatment in patients with severe asthma and should contribute to future informed decisions about this treatment. In Portugal, although four studies described outcomes of omalizumab treatment in real-life settings,5, 6, 7, 8 only one is prospective and it was conducted in a single healthcare unit.
The aim of this prospective multicentre study was to assess the clinical effect of omalizumab in Portuguese patients with severe persistent allergic asthma, specifically concerning asthma control and exacerbations.
MethodsDesignThis was a multicentre, descriptive, observational, prospective study, conducted during routine asthma care. The target population was individuals treated with omalizumab for severe persistent asthma.
Setting and participantsThe study was conducted in seven Pulmonology and Allergology departments from six hospitals in north and centre mainland Portugal. In each unit, all asthma patients under treatment with omalizumab were included, regardless of the age, length of previous therapy with omalizumab or actual treatment schedule. All patients had omalizumab treatment approved by the therapeutic commission and hospital administration, as required in Portuguese hospitals. Minimal criteria for approval of omalizumab treatment for asthma are uncontrolled, severe persistent, allergic asthma, with frequent exacerbations.
Data collectionData were collected at each routine visit for omalizumab administration, during 12 consecutive months. In the participating centres, omalizumab was administered at 2- or 4-week intervals with doses based on IgE serum levels and body weight, as recommended by EMEA – European Medicines Agency.9 A structured form, to be filled by both the patient and the nurse responsible for omalizumab administration, was developed in order to standardize data collection at the different study sites. This form included questions on asthma worsening, medication intake, healthcare resources utilization and work/school absenteeism; information on side effects was possibly related with the previous treatment administration. The Control of Allergic Rhinitis and Asthma Test (CARAT)10, 11 was also part of the form.
Data collection spanned from January 2011 to December 2013 in the different departments (Additional file 1).
OutcomesAsthma and rhinitis control was defined by CARAT global score >24. CARAT lower airways score ≥16 and upper airways score >8 were the cut-off values for control of, respectively, the lower and upper airways only.
Asthma exacerbation was defined as having unscheduled healthcare utilization or increases in OCS intake because of asthma. Asthma worsening, unscheduled healthcare utilization and increases in oral corticosteroid intake were based on a positive answer to the questions: “In the last 3 days, have you felt your asthma getting worse?”; “Since the last administration, have you had any unscheduled healthcare visit or emergency room visit because of your asthma?” and “In the last 3 days, did you need to start or increase oral corticosteroid intake?”, respectively.
Statistical analysisStatistical analyses were performed using IBM SPSS Statistics version 21 (2012 SPSS Inc., IBM Company, Chicago, US). Categorical and continuous variables were analyzed using descriptive statistics as appropriate. Survival analysis was used to assess the time until the first exacerbation. Generalized Estimating Equations, taking into account the multiple measurements in this longitudinal design, were used to assess variations in control scores over time; a Wald Chi-square test with a p-value of <0.05 was considered statistically significant. As CARAT evaluates a 4-week period, control scores were analyzed for visits with at least a 4-week interval.
ResultsA total of 48 adults with severe persistent allergic asthma were included (Table 1). Patients were under treatment with omalizumab for between 0 and 67 months, most for more than 3 years (median, 45 months). Most patients (n = 31, 65%) were prospectively followed for 12 months and five were followed for less than 9 months (Additional file 1). During the study, the median (min–max) number of medical visits for each patient was 14 (7–28). Side effects were reported in 5% of the visits (Additional file 2); thirty-two (67%) patients did not report any side effect during the study.
Table 1. Characteristics of the study population (n = 48).
| Female (n, %) | 36 | 77 |
| Age (mean, SD) | 52 | 10 |
| Hospital units (n, %) | ||
| Centro Hospitalar e Universitário de Coimbra, EPE – Pulmonology | 15 | 31 |
| Centro Hospitalar São João, EPE – Allergology | 12 | 39 |
| Centro Hospitalar São João, EPE – Pulmonology | 8 | 26 |
| Centro Hospitalar do Porto – Allergology | 6 | 13 |
| Centro Hospitalar de Trás-os-Montes e Alto Douro – Pulmonology | 4 | 8 |
| Hospital Pedro Hispano – Allergology | 2 | 4 |
| Centro Hospitalar do Alto Ave – Allergology | 1 | 2 |
| Follow-up period, months (median, min–max) | 12 | 3–12 |
| Visits with reported OCS intake (mean% per patient, SD) | 15 | 32 |
SD – standard deviation; OCS – oral corticosteroid.
CARAT scores were analyzed for visits with at least a 4-week interval in a total of 414 visits. During the study period, asthma was controlled in 34% of the visits; the mean (SD) CARAT score was 20.4 (7.5). There was no statistically significant variation in any of the CARAT mean scores during the 12-month follow-up period (p > 0.05).
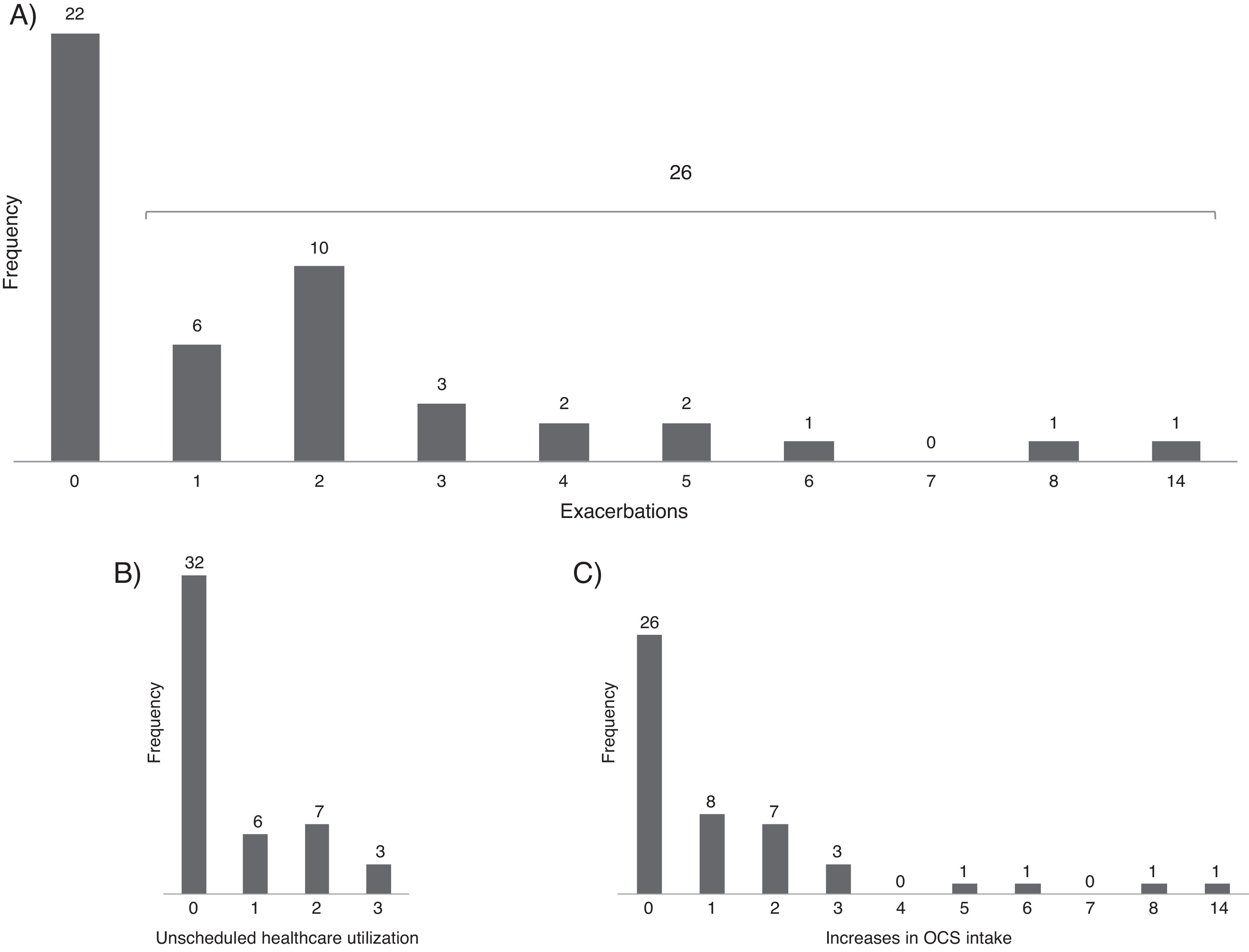
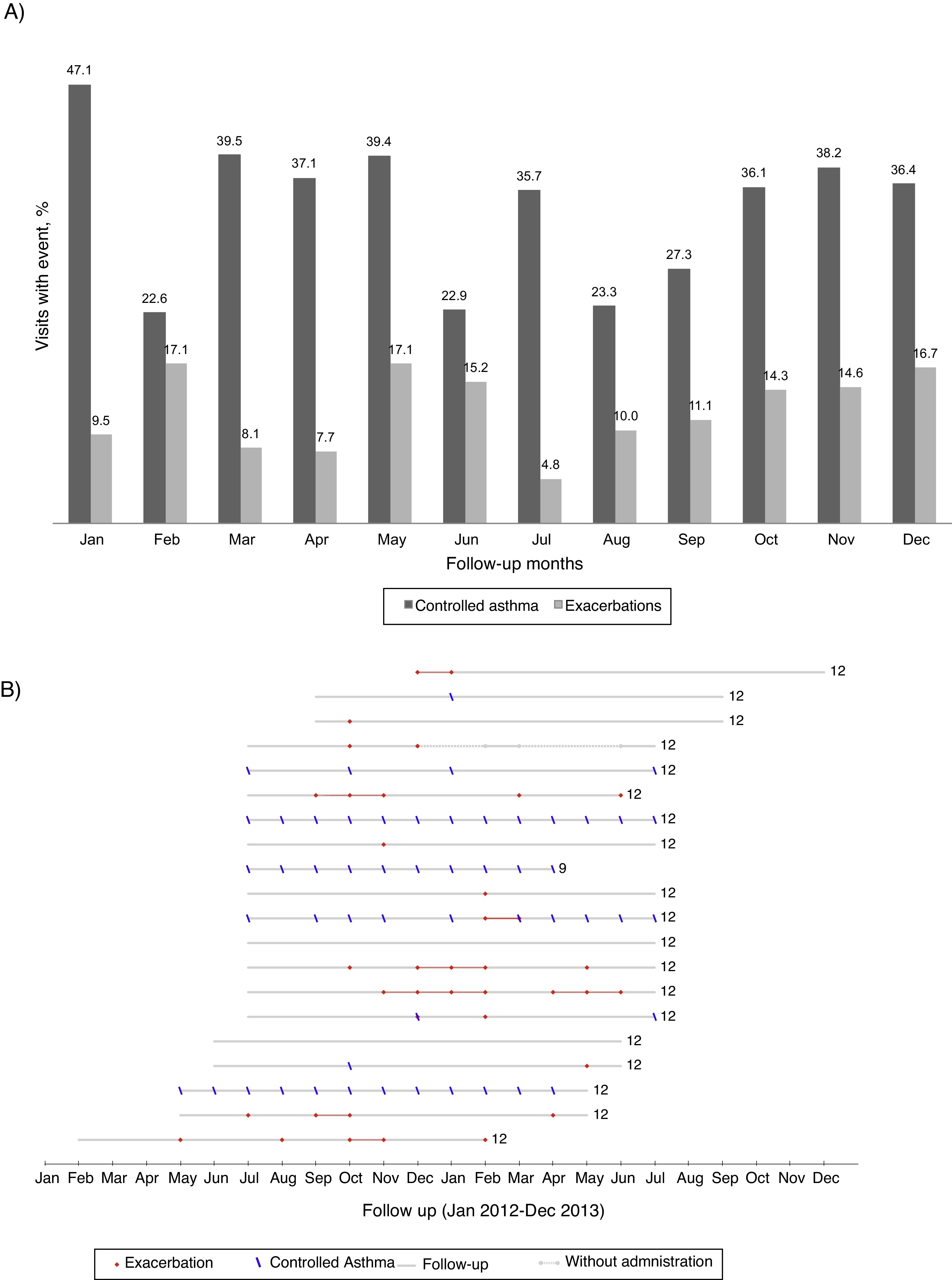
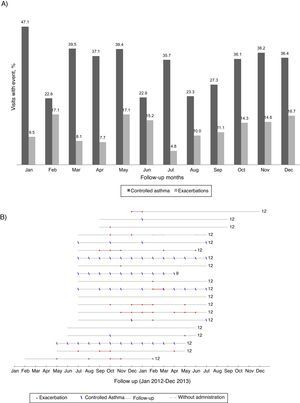
Asthma exacerbationsDuring follow-up, 26 patients (54%) had at least one asthma exacerbation (Figure 1); the 12-month rate of asthma exacerbations per patient was 1.7–1.3 increases with OCS intake and 0.6 unscheduled healthcare utilizations. The first exacerbation occurred on average (mean [95% CI]) 7.2 (5.9–8.6) months after entering the study. There was no clear pattern of distribution of asthma exacerbations and control throughout the year (Figure 2).
Figure 1. Distribution of the number of exacerbations (A) unscheduled healthcare utilization (B) and increases in OCS intake (C) in the 12-month follow-up period.
Figure 2. (A) Percentage of visits reporting controlled asthma and exacerbations according to the month of the year (all patients included). (B) Reports of controlled asthma and exacerbations during follow-up, per patient. In (B) numbers on the right indicate total months of follow-up; continuous grey lines represent the follow-up period and dashed grey lines interruptions in omalizumab treatment; red dots represent visits with report of exacerbation and blue dashes visits with CARAT global score >24–data from the 20 patients treated with omalizumab in Centro Hospitalar São João, EPE.
Thirty-three (69%) participants reported at least one period of worsening of asthma symptoms without needing unscheduled medical care or OCS intake. Eight (17%) had more than four periods of worsening of asthma.
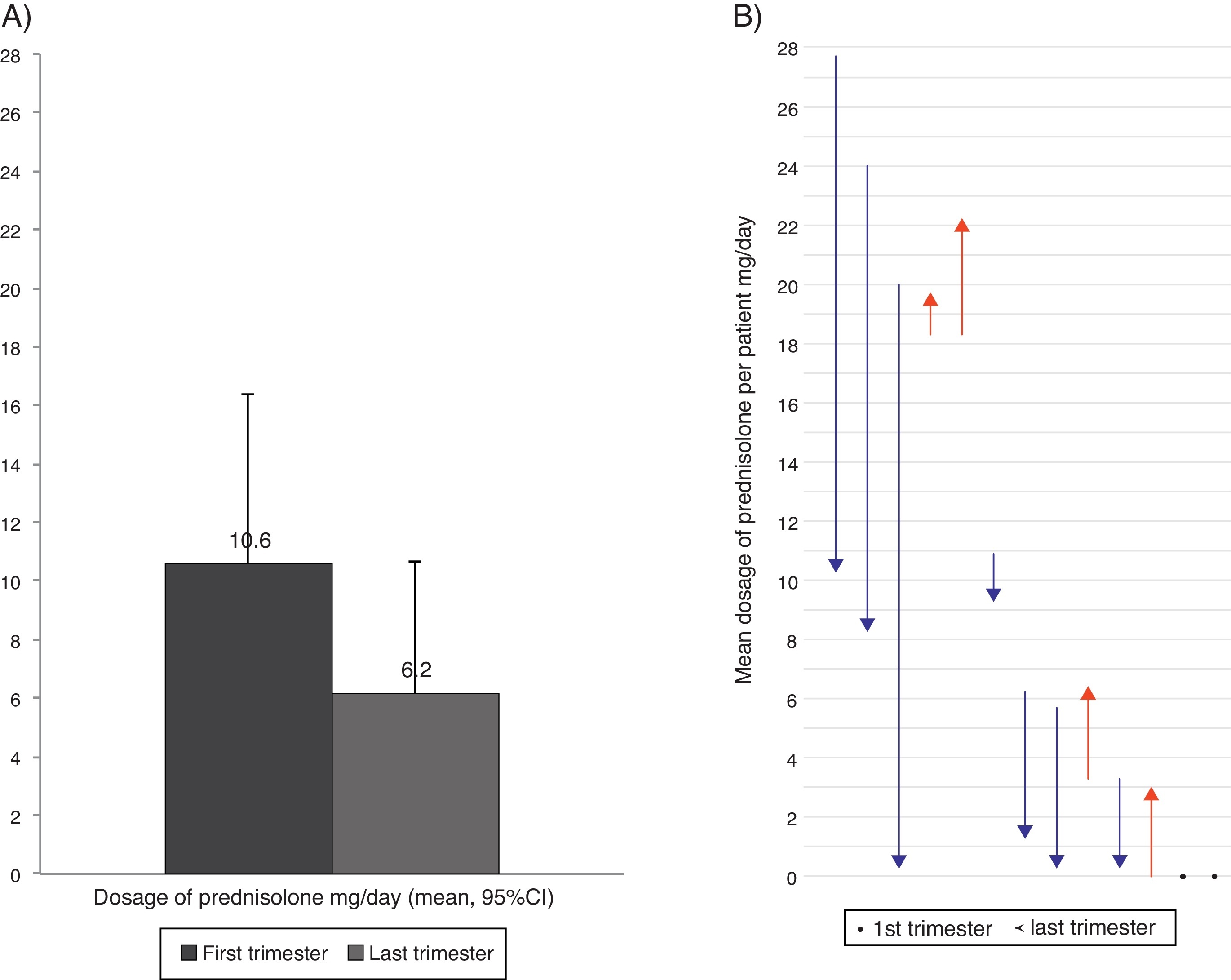
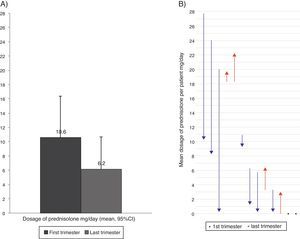
Oral corticosteroids intakeFourteen patients (29%) took OCS because of asthma at least once during the follow-up. Seven patients were taking daily OCS in the first visit and five of them took OCS during the full length of the study, in a mean (SD) dosage of 18.9 (4.4) mg prednisolone/day. Comparing the first and the last trimesters of the study, seven patients reduced the daily dosage of OCS, four patients reported a dosage increase, and two had no variation. Overall, the daily dosage of OCS had a non-significant reduction of 41.6% (Figure 3). One patient had a follow-up inferior to two trimesters and was not considered for comparison purposes.
Figure 3. Mean dosage of oral corticosteroids intake in the first and last trimester of the follow-up year (considering the 13 patients with OCS intake), (A) Overall reduction (B) Per patient. In (B) arrows represent the dose variation from the first to the last trimester; red lines represent increases and blue lines decrease in the dosage of oral corticosteroid intake; and dots represent no variation in the dosage.
DiscussionDuring the 12-month follow-up, patients with severe persistent allergic asthma treated with omalizumab had their asthma controlled in 34% of the visits; 2/3 had no need for unscheduled medical care due to asthma and 46% remained exacerbation free. The asthma exacerbation rate was 1.7 exacerbations per patient per year (the rate of treatment courses with OCS was 1.3 and of unscheduled healthcare utilizations was 0.6).
Previously published observational studies performed in real-life settings reported that omalizumab is effective in the treatment of patients with severe persistent allergic asthma.5, 12, 13, 14, 15, 16, 17, 18
The present study showed that the proportion of patients with good asthma control in these patients with severe persistent allergic asthma was stable. The asthma control score did not change significantly during the follow-up period probably because patients were already under treatment with omalizumab at enrolment. This ‘stability’ concurs with several studies5, 16 that reported improvements in asthma control in the first 16 weeks of omalizumab treatment and a tendency to stabilize the effect in the following months. Previously published studies using CARAT questionnaire to assess the control of asthma and rhinitis in other settings found a mean (SD) CARAT score of 17.8 (6.4) in a community of inner Portugal,19 17.8 (0.2) in patients followed in the Allergology Department of a University Hospital20 and 17.2 (6.7) in patients referred to the Allergy outpatient clinic from a district hospital.21 The mean CARAT score found in the present study for patients with severe asthma is similar to those found in patients with non-severe asthma, suggesting a positive effect of omalizumab treatment.
The rate of 0.6 emergency visits per patient-year observed in the present study is inferior to the rates reported by Molimard and co-workers – 1.1 emergency visits per patient-year after a follow-up of more than 5 months,18 and by Cazzola and co-workers – 1.2 visits per patient-year after a follow-up of 12 months.12
The exacerbation rate observed in the present study is lower than the rates reported in France18 and in the PERSIST study16 but greater than those of Germany,17 Italy,12 Ireland14 and Spain,15 in similar studies. Overall, in our study, the frequency of asthma exacerbations during one-year omalizumab treatment was lower than expected for these severe patients.22 However, in spite of being treated with omalizumab, five patients had ≥5 exacerbations. We could speculate whether before starting omalizumab the asthma control in these patients was even worse or if the treatment with omalizumab was ineffective and should be discontinued.
Previous work found that omalizumab had a marked effect in the reduction of seasonal peaks of asthma exacerbations, with the monthly exacerbations rate remaining the same throughout the year.23 Our study seems to support these results, as no pattern of seasonal asthma exacerbations was evident throughout the year in these patients treated with omalizumab.
The reduction of OCS intake shown in this study is in accordance with previous reports.7, 8, 12, 18
Our study was the first multicentre real-life study on the effect of omalizumab in patients with severe persistent allergic asthma in Portugal. This study has several limitations. Firstly, at enrolment, patients were already using omalizumab as add-on therapy and no comparable prior data were available. Moreover, in one-third of the patients it was not possible to access the registries for the complete 12-month treatment period.
ConclusionsThis multicentre study showed that Portuguese adult patients with severe persistent allergic asthma under treatment with omalizumab, achieved asthma control in 34% of the medical visits with only 1/3 requiring unscheduled or Emergency Room care because of asthma exacerbations, supporting the maintenance of the clinical effect during treatment with omalizumab in real life. Further prospective studies in real-life Portuguese settings are needed to assess the reduction in corticosteroids intake in patients starting omalizumab therapy. These studies should be useful for a grounded pharmacoeconomic evaluation of omalizumab treatment in Portugal.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interest.
Acknowledgements
We thank all members of the REAG (Rede de Especialistas em Asma Grave) specially Laura Simão, Aurora Carvalho, José Ferreira, Rui Silva, António Costa, António Antunes, Eugénia Almeida, Isabel Pereira and Luísa Barata who actively participated in study set-up and discussion of results. We also thank Ângela Lopes for data insertion and Orquídea Ribeiro, from the Biostatistical consulting team from CIDES (Health Information and Decision Sciences Department), for the support in data analysis. Finally we thank Novartis for an unrestricted grant that supported data processing and analysis.
Appendix A. Supplementary materialSupplementary material associated with this article can be found in the online version available at doi:10.1016/j.rppnen.2015.03.002.
Appendix A. Supplementary materialThe following are the supplementary material to this article:
Received 30 October 2014
Accepted 8 March 2015
Corresponding author. fonseca.ja@gmail.com