The numbers of patients needing prolonged mechanical ventilation are growing. The rehabilitation programs to be implemented in specialized inpatient facilities are ill defined. There is a clear need to establish guidelines to define the optimal rehabilitation program in this setting. In this article we review the current evidence and propose some guidance.
According to the NAMDRC consensus conference, prolonged mechanical ventilation (PMV) defines patients who require at least 6 h of mechanical ventilation for >21 consecutive days.1 Recent estimates indicate that in the US the numbers of PMV are expected to double by the year 2020, reaching more than 600,000 patients.2
The reasons for this are heterogeneous: a greater capacity of ICUs to assist severe respiratory failure; modulate systemic inflammatory response syndrome and severe sepsis in patients with a high prevalence of secondary neuromuscular dysfunction and severe physical deconditioning, all these at increasingly advanced ages. Another large group of patients is represented by those suffering from severe injuries to the central nervous system or incurable and progressive neuromuscular diseases.
Currently we only have partial information about the functional outcomes and quality of life of these patients, who are now described as chronic critically ill patients.3 Their life trajectories, during their stay at long-term acute care hospitals, or LTACs or after a successful ventilatory weaning, are yet to be described. Many will have severe, permanent cognitive and physical impairments and serious limitations due to their disability, which will obviously involve high psychosocial costs.4
Treating these patients in specific venues with strong rehabilitative focus is normally recommended, with the cost savings and higher ventilator weaning rates.5
PMV patients (also referred to as Chronically Critically Ill patients) are very demanding due to multiple systems and organs dysfunctions. Beyond prolonged dependence on mechanical ventilation, muscle, neuro-endocrine, skin and brain dysfunctions give way to a distinct and complex syndrome.3
Although liberation from the ventilator will be one of the most important interventions in this setting, there is more than one type of weaning.
Psychological factorsPsychological factors (like depression or delirium) may hinder ventilator weaning.6 Sleep deprivation or unrecognized sleep disorders may also interfere with weaning.7 So measures to improve sleep and psychological disorders can impact other outcomes.8 In fact educational interventions, counseling and stress management can decrease the risk of developing psychological disorders. Moreover, effective pharmacological treatment of anxiety and depression is also mandatory.
Swallowing dysfunctionOropharyngeal dysphagia (OPD) has been described in patients with PVM for three decades, and yet, has not received much attention in research addressing different subgroups of this diverse population.9, 10 Swallowing dysfunction is a common complication in chronic critically ill patients, which affects nearly half of the non-neurologic patients requiring percutaneous dilatational tracheostomy and almost all of those with neurologic involvement; it is well known to deteriorate outcomes, and delay the weaning and tracheostomy decannulation process.11 A number of possible specific swallowing dysfunctional conditions arise in PMV patients; to mention a few, sarcopenic dysphagia, presbyphagia, neuromuscular dysfunction related dysphagia and even with some structural swallowing disorders.12 Overall evaluation measures and treatment are based on experiments with different methodologies13, 14 but not on controlled studies which consider the unique pathophysiology of chronic critically ill patients.15 It is worth mentioning the potential role of fiberoptic endoscopic evaluation of swallowing (FEES) as an objective tool to precisely classify and guide therapeutic interventions after prolonged intubation and in tracheostomy patients. In fact, FEES with sensory testing is improving the rehabilitation designs and protocols.16
The main components of a dysphagia rehabilitation program include oral health care, swallowing rehabilitative techniques, and food consistency modification. To the best of our knowledge there are no detailed studies or any evidence about the most effective strategies to improve the swallowing process in different non-invasive and invasive PMV scenarios. This role which has been suggested for FEES as an objective tool to precisely classify and guide therapeutic interventions after prolonged intubation and in tracheostomy patients should be considered.17
Skin integritySkin integrity is an independent determinant of survival in patients requiring prolonged mechanical ventilation and is a potentially modifiable factor.18 The most important risk factors for development of pressure ulcers while in ICU are total score on Braden Scale, mobility, activity, sensory perception, moisture, friction/shear, nutrition, age, blood pressure, length of stay, APACHE II, vasopressor administration, and co-morbid conditions.19 Where it is chronic, central neurological involvement and small fiber pathology (which explains chronic sensory impairment and pain in neuro-critical care survivors) are the main features.20
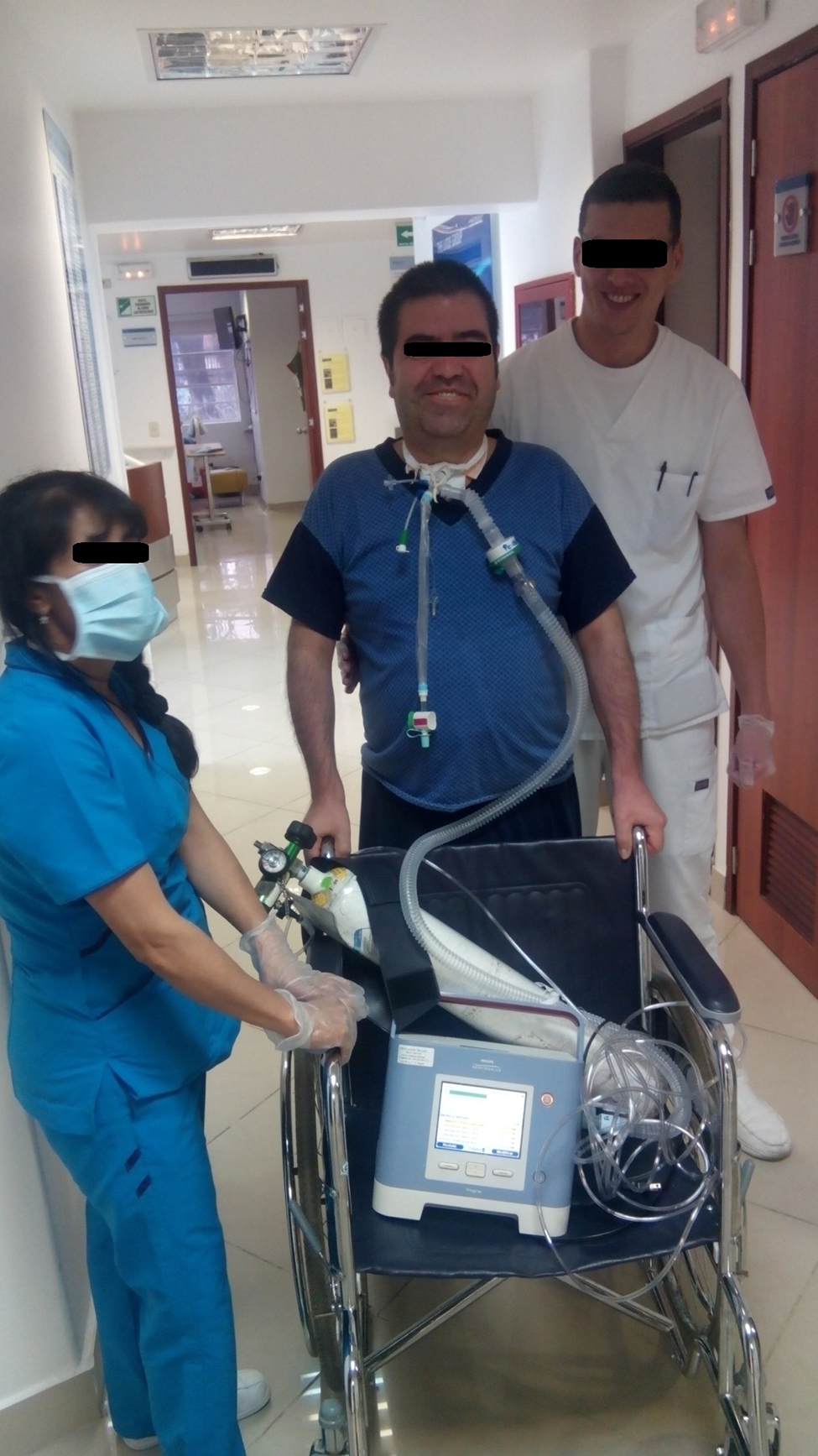
Whole body rehabilitation ( Fig. 1 )Increasing evidence supports early physiotherapy for the critically ill patient21, 22.
Figure 1. A ventilator-dependent tracheostomized patient walking with assistance from the staff.
RCT in ICU settings have already shown that early physical and occupational rehabilitation of mechanically ventilated patients translates into better weaning outcomes.23
In weaning facilities the studies evaluating the impact of rehabilitation in prolonged mechanical ventilated patients (Table 1) show positive results, from increase in limb strength24, 25 to improvement in functional measures like FIM,24, 25, 26, 27, 28 respiratory muscle strength25 and mechanics.27 A RCT study has also shown a significant survival benefit in the rehabilitation group.26 In these studies mortality ranges from 12%24 to 30%25 and weaning rate from 47%29 to 98%.24 The studies include also very different sub-groups of patients from chronic respiratory diseases25, 26, 27, 28, 29, 30 to post-operative and acute lung injuries.24, 25, 26 Only one study includes neuromuscular patients.28 Even in RCT, rehabilitation programs are not uniform, with some studies proposing 6-week long programs25, 26 while others proposing shorter times27, 30; some include specific inspiratory muscle training,24 a few others formal cardiopulmonary endurance exercise27 and the others use electrical stimulation.29
Table 1. Summary of retrospective/prospective/RCT in weaning facilities.
| Article | Number of patients | Diagnosis | Design | Intervention | Outcome | Frequency of exercise sessions | Results |
| Martin 24* | 49 | Pneumonia, CHF, ARDS | Retrospective | Whole body Rehab, IMT | Weaning success, FIM, MRC | 5days/wk 30–60 min (1–2 sessions/day) | ↑ limbs strength; ability to sit to stand and supine to sit; FIM Mortality 12% Weaning rate 98% |
| Chiang 25** | 39 | Chronic lung disease, Post-op, ALI | RCT | Whole body Rehab, Diaphragmatic breathing | Barthel Index, FIM, ventilator free time, Pimax, Pemax, Dynamometer | 5 days/wk for 6 wks | ↑ limbs strength; Pimax, Pemax FIM, BI Ventilator free time Mortality 17.6% Weaning rate 47% |
| Chen 26** | 34 | Chronic lung disease, Post-op, ALI | RCT | Whole body Rehab, Diaphragmatic breathing | 1-year Survival FIM | 5days/wk for 6 wks + 1day/wk for 6 wks | ↑ FIM, survival Mortality 30% Weaning rate (at 1 year) 36% |
| Clini 30* | 77 | COPD, Post-Op | Prospective | Whole body Rehab (including pedaling and weights holding) | ADL, survival weaning success | 6days/wk for 15 sessions | ↑ ADL Mortality 13% Weaning rate 74% |
| Chen 27** | 27 | COPD/Chronic Lung Disease, CHF, SCI, Pneumonia | RCT | Whole body Rehab, Cardiopulmonary endurance exercise | Pulmonary mechanics, Barthel Index, FIM, weaning success, mortality | 10 exercise training sessions | ↑ VT, RSBI, FIM Mortality 0% Weaning rate 75% |
| Montagnini 28 | 56 | COPD, NMD, Trauma, CHF, Post-op | Retrospective | Whole body Rehab (including bed-side cycle ergometer) | FIM | 6 days/wk | ↑ FIM Dyspnea (MRC) PaO2/FiO2 ↓ PaCO2 |
| Vitacca 29* | 240 | COPD, ARF, Neurological diseases, Post-op | Prospective | Whole body Rehab (including electrical stimulation) | Barthel Index, Gussago Nursing Scale, survival, weaning success | 6 days/wk 1 h/day (in 1 or 2 30 min sessions) | ↑ BI, GNS, DPAP PaO2/FiO2 ↓ PaCO2 ↓ Borg Mortality 13.8% Weaning rate 47% |
CHF, chronic heart failure; FIM, functional independence measure; IMT, inspiratory muscle training; BI, barthel index; Post-op, post-operative; ALI, acute lung injury; SCI, spinal cord injury; NMD, neuromuscular disorders; ARF, acute respiratory Failure.
* Excluded NMD.
** Excluded neurological disorders.
Type of intervention, frequency, duration and intensity based on Denehy et al.31 and Hanekom et al.32 is proposed in Table 2.
Table 2. Type of intervention, frequency, duration and intensity.
| Frequency of exercise sessions | 15 min/day (if <4 h spontaneous breathing) 2× 15 min/day (if >4 h spontaneous breathing) | |
| Type of exercise | Marching in place Moving from sitting to standing Arm and leg active and active resistance movements | Based on 34 Active range of motion exercises for all major joints, bed mobility exercises (e.g. lateral rolling, supine to sit), dangling at the edge of the bed, postural retraining, balance exercises (e.g. reaching in and out of the base support, challenges to elicit “righting” reflexes), training in ADL (eating or simulated eating, grooming, bathing, dressing, and toileting), transfer from seated to a standing position and from bed to chair or commode, standing exercises such as reaching in and out of the base of support, mini-squats, marching, and ambulation (with or without assistive devices) |
| Intensity | Target modified Borg Scale score 3–5 | |
To improve survival, diminish co-morbidities, decrease ventilator dependence, improve functional status and health related quality of life, decrease hospital re-admissions, decrease length of stay in long-term care, favor return to work and social re-integration and reduce the amount of ineffective care. Caution must be taken about potential contraindications (Table 3).
Table 3. Contraindications of physical therapy in PMV.
| Hemodynamic instability (in this case the patient should be discharged to an ICU) |
| Non-controlled behavioral disorders (in this case also the patient should be discharged) |
| Severe anemia (less than Hgb 7 g/dl) or thrombocytopenia (Platelets <40–50.000/dl). In Hemato-oncologic patients levels between 20 and 30.000 can be accepted |
| HR <60 or >110/min (or >30 bpm above resting predicted HR); also consider underlying cardiac disease |
| Mean ABP <65 mmHg or >200 mmHg |
| Sepsis or persistent fever (>38 °C) (in fever of central cause or while fever cause is being investigated, between fever peaks, consider lower intensity/passive interventions) |
| End-stage patients included in palliative care |
Abbreviations: ICU, intensive care unit; Hgb, hemoglobin; HR, heart rate; ABP, arterial blood pressure.
There has been a growing interest in different rehabilitative strategies to treat PMV patients. However most of the published studies covered non-randomized clinical trials.
In Table 4 we review some of the most interesting topics involved in these interventions. Cognitive training has been tested among post-ICU survivors and preliminary data are encouraging33 and a new RCT is being designed.34 A prospective non-randomized study has also shown that early intra-ICU clinical psychologist intervention may help critically ill trauma patients recover from post-traumatic stress disorder.8
Table 4. Evidence-based multidisciplinary rehabilitative interventions.
| Cognitive rehabilitation | 8,33,34 |
| Feeding/nutrition and swallowing | NA |
| Sleep | 35 |
| Respiratory – respiratory muscle training Secretion management; Weaning/decannulation protocols | 36 37 38,39 |
| Physical therapy – limb exercises, exercise training, neuromuscular electrical stimulation | See Table 1 |
| Co-morbidities management (e.g. CHF, DM, COPD), | NA |
| Skin care | NA |
Abbreviations: CHF, chronic heart failure; DM, diabetes mellitus; COPD, chronic obstructive pulmonary disease.
As mentioned before no RCT so far has analyzed swallowing dysfunction management.
Disruption of sleep is very common in mechanically ventilated patients. In an observational study Koldobskyi et al. have shown that specific facilities (like LTAC's) maintain the patients’ circadian rhythm compared to the ICU environment.35
In a recent meta-analysis, examining three trials involving a heterogeneous group of patients, inspiratory muscle training was found to significantly increase inspiratory muscle strength in adults undergoing mechanical ventilation.36 Further research is needed including more homogeneous PMV populations and analyzing more relevant outcomes.
Concerning airway secretion management, Gonçalves et al. showed in a RCT that adding mechanical in-exsufflation improved extubation outcomes, increasing the efficacy of NIV post-extubation.37
There are few RCT in the context of weaning tracheostomized patients. Hernandez showed that deflating the tracheal cuff in tracheostomized patients shortens weaning, reduces respiratory infections, and probably improves swallowing.38 Jubran et al. in patients requiring PMV, showed that unassisted breathing through a tracheostomy (compared with pressure support) resulted in shorter median weaning time.39
Verceles et al. have shown that in patients under PMV, higher comorbidity burden is associated with increased risk of transfer to acute care.40 In fact, in a retrospective study, Schulman et al. suggest that tighter glycemic control was associated with better outcomes in CCI patients. Prospective studies addressing the importance of co-morbidities treatment optimization are warranted in this setting.41
Members of the team and roles ( Table 5 )Teamwork has been shown to improve outcomes. In fact medical ICU nurses’ reports of collaboration were associated positively with patient outcomes.42
Table 5. Members of the team and roles.
| Core team: MD, Nurse, RT |
| MD: Serve as a coordinator |
| Nurse: Liaise with RT/PT as required |
| Speech pathologist: Liaise with RT/PT as required to evaluate swallowing |
| Occupational therapist, psychologist, nutritionist/dietitian, social worker involved as required |
The core team is normally the medical doctor, the nurse and the respiratory therapist (or specialized physiotherapist); the sense of interdependence exists between each other to ensure workflow is efficient, effective and coordinated.43 Daily multidisciplinary rounds integrate the different knowledge to promote the clinical goals and formulate a plan of interventions. Coordination between professionals is essential to make a personalized and integrated therapeutic plan.
The speech pathologist has a very important role not only to facilitate speech in patients with tracheostomy but also to evaluate swallowing.44
Due to the high resource use and costs involved in supporting ventilator-dependent patients, social workers are indispensable to assure effective integration of health, social, education and employment services and voluntary and independent sectors when pertinent.45 It also plays a fundamental role avoiding any possible discrimination that may rise from the disability surrounding PMV. Frequent communication with the family with discussion about realistic versus futile goals is also an important goal.
Evaluation of efficacyThe traditional severity-of-illness scoring systems like the SOFA (Sepsis-related organ failure Assessment) score or the APACHE III were developed in the acute Intensive care Unit (ICU) population.46 These systems were not established or validated using populations of patients under PMV (or also referred to as Chronically Critically Ill patients), as a result caution should be used when interpreting such data.47
Therefore the development of adequately performing severity-of-illness measures appropriate to this patient population is highly needed.
In the absence of newer multidimensional tools, the majority of authors use the Functional Independence Measure (FIM) to evaluate the efficacy of a rehabilitation program.24, 25, 27, 28 They evaluate the functional status of the patient at admission, after half the length of the program, at the end of the program and at discharge. If the results are not improving significantly the program/interventions should be adjusted on a regular basis, decreasing/increasing or maintaining intensity based on the eventual resolution of contraindications/significant improvement/deterioration/plateauing. To make this choice the Minimal Detectable Change (MDC) or Minimal Clinically Important Difference (MCID) of the disability scoring system being used should be considered.48 For stroke patients the MCID for total FIM score was 22 points.48 In studies including PMV patients the improvement in total FIM score ranged from 1125 at 6 weeks to 44 points at 1 year.26
Eventually after >1 month of structured multidisciplinary approach, consider transferring the patient to another setting (home or nursing home) if no improvement is gained.
ChallengesIn one facility with more than 30 patients with heterogeneous diagnosis, the logistics of tailored therapeutic interventions can be very complex, from timing and chronobiology to coordination. To maintain a constant flow of interventions a highly flexible team is needed.
The role of the family/non-professional caregivers to support and assist in the rehabilitation program can have a role in improving functional outcomes and potentiate team interventions.
It is possible that some subgroups of PMV/CCI benefit more from whole body rehabilitation than others. A challenge for the future will be identifying which subgroups of PMV/CCI benefit most from these programs.
ConclusionsUntil now, post-ICU patients have not had a recognized rehabilitation program and the care process is still fragmented. There is a clear need to establish guidelines to define the optimal rehabilitation program in this setting. Integrated, multidisciplinary rehabilitation programs for ventilator-dependent chronic critically ill patients should be urgently defined. Randomized studies for this situation are welcomed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Received 18 March 2015
Accepted 24 March 2015
Corresponding author. jcwinck@mail.telepac.pt