Expression of ERCC1 has not been well described in fine‐needle aspiration biopsies (FNABs) in patients with non‐small cell lung cancer (NSCLC). We investigated the expression of ERCC1 in correlation with EGFR expression and clinicopathological factors in patients with NSCLC in order to determine if these play a role in the prognosis of the disease.
MethodsWe studied 45 patients, 34 with adenocarcinoma and 11 with squamous cell carcinoma. Of these 45 patients, 35 were males and 10 females, aged between 45 and 83 years, 30 smokers and 15 non‐smokers. Eighteen (18) tumors were of stage I, twelve (12) stage II and fifteen (15) stage III. To investigate the expression of ERCC1 and EGFR (scores 0, 1, 2, 3), immunocytochemistry was performed on air dried specimens (FNABs) using monoclonal antibodies by alkaline‐phosphatase (APAAP) method.
ResultsERCC1 expression was detected in tumors from 27 patients (60%) and EGFR in 10 patients (22.2%). ERCC1 was expressed more frequently in males (65.7%) in patients >65 years old (64%), in smokers (66.7%) and in stage I (66.7%). Negative ERCC1 expression was significantly associated with the presence of EGFR. EGFR was expressed only in adenocarcinomas and more frequently in women (70%) and non smokers (53.3%).
ConclusionsERCC1 expression was identified as positive (scores 2+ and 3+) in the majority of NSCLCs and seems to be an independent prognostic marker of longer survival. In addition EGFR expression was positive (scores 2+ and 3+) in the minority of NSCLCs and only in adenocarcinomas, more frequently in ERCC1‐negative (scores 0 and 1+) tumors, suggesting that it is not an independent prognostic marker for the outcome of the patients suffering from NSCLC.
A expressão de ERCC1 não foi ainda suficientemente descrita em biópsias aspirativas por agulha fina (FNAB) em doentes com carcinoma pulmonar de não pequenas células (NSCLC). Investigámos a expressão de ERCC1 em correlação com a expressão EGFR e os fatores clinicopatológicos em doentes com NSCLC para determinar se estes desempenham um papel no prognóstico da doença.
MétodosEstudámos 45 doentes, 34 com adenocarcinoma e 11 com carcinoma de células escamosas. Desses 45 doentes, 35 eram homens e 10 mulheres, com idades entre os 45‐83 anos, 30 fumadores e 15 não fumadores. Dezoito tumores encontravam‐se no estádio I, 12 no estádio II e 15 no estádio III. Para investigar a expressão de ERCC1 e EGFR (resultados 0, 1, 2, 3), foi realizada imunocitoquímica em espécimes a seco (FNAB), usando anticorpos monoclonais pelo método de fosfatase alcalina (APAAP).
ResultadosA expressão ERCC1 foi detetada em tumores de 27 doentes (60%) e a do EGFR em 10 doentes (22,2%). O ERCC1 foi expresso com maior frequência em homens (65,7%), em doentes com mais de 65 anos (64%), em fumadores (66,7%) e no estádio I (66,7%). A expressão ERCC1 negativa foi significativamente associada à presença de EGFR. O EGFR foi expresso apenas em adenocarcinomas e com maior frequência em mulheres (70%) e não fumadores (53,3%).
ConclusõesA expressão de ERCC1 foi identificada como positiva (resultados 2+ e 3+) na maioria dos NSCLC e parece ser um marcador de prognóstico independente de maior sobrevivência. Além disso, a expressão de EGFR foi positiva (resultados 2+ e 3+) numa minoria dos NSCLCs e apenas em adenocarcinomas, com maior frequência em tumores ERCC1‐negativos (resultados 0 e 1+), sugerindo que não é um marcador de prognóstico independente na evolução de doentes que sofram de NSCLC.
Primary lung cancer is the leading cause of cancer death in many countries.1,2 Approximately 80% of primary lung cancers are non small‐cell lung carcinomas (NSCLCs). Surgical resection is considered to be a curative treatment during earlier stage and adjuvant chemotherapy or radiotherapy in more advanced stage of the disease.
Excision repair cross‐complementation group 1(ERCC1) is one of 16 genes that encode the proteins of the nucleotide excision in repair complex.3,4 This multiprotein complex also links the DNA repair process with other cellular processes such as DNA transcription.
Different studies have already reported the expression of ERCC1 in human ovarian cancer cells in vitro5 in primary gastric adenocarcinomas6 in colorectal cancer7 in esophageal cancer8 and in non small cell lung cancer (NSCLC).9
Epidermal growth factor (EGFR) is a 17kDa transmembrane glycoprotein which can bind and become activated by various ligands including epidermal growth factor (EGF), transforming growth factor alpha (TGFa) and certain virally encoded growth factors. EGFR overexpression is found in a variety of neoplasms such as malignant gliomas, breast and lung carcinomas. Among lung cancers, overexpression is largely due to increased transcription and is most prevalent in lung squamous cell carcinomas. Increased overexpression has also been observed in adenocarcinomas of the lung and large cell carcinomas but not in small cell carcinomas.10 The aim of this work is to clarify and validate, in a retrospective study, the prognostic relevance of ERCC1 expression correlated with EGFR expression and other clinicopathological variables, using immunocytochemistry in cytological material (FNAB) of patients with non small cell lung cancers (NSCLCs).
Materials and methodsForty‐five patients (45) with operable NSCLC were diagnosed and studied cytologically on FNABs and had their diagnoses confirmed histologically after the operation. Diagnoses were established by both morphology and immunocytochemistry. Objective and measurable cytomorphologic differences between squamous cell carcinoma and adenocarcinoma exist. The aspirated cancer cells in adenocarcinoma are found in clusters or singly. The cytoplasm is finely vacuolated and faintly stained with indistinct cell borders. The nuclei have a delicate chromatin pattern and prominent nucleoli. In contrast tumor cells of squamous cell carcinoma have sharp cell borders, dense cytoplasm, nuclear hyperchromasia, and significant nuclear membrane irregularities. Spindle cell morphology and “tadpole” cells are commonly seen. However cytomorphologic differences between the two types are not always consistent. Immunocytochemistry has a significant role as an adjunct study in this context. In our settings we performed immunocytochemistry on cell block material with antibodies against TTF‐1 (clone 8G7G3/1, Dako, M3575), CK7 (clone OV‐TL, Dako, M7018), CK20 (clone K20.8, Dako, M7019), P63 (clone 4A4, Dako, M7247), CK5/6 (clone D5/16/B4, Dako, M7237), with the dilutions of 1:50, 1:200, 1:30, 1:60, and 1:10 respectively. Immunostaining was performed on the Ventana automated system. Thyroid tissue was used as the control for TTF‐1, benign lung for CK7, colon cancer for CK20, benign prostate for P63, and skin for CK5/6. Immunostaining was evaluated semi‐quantitatively for both extent and intensity and was defined as negative if no staining was present, low if staining was present in less than 10% of tumor cells, moderate if staining occurred in 10–50% of tumor cells, and high if staining was seen in >50% of tumor cells. Adenocarcinomas were TTF‐1 positive, CK7 positive, CK20 negative, P63 negative, and CK5/6 negative. Squamous cell carcinomas were TTF‐1 negative, CK7 negative, CK20 negative, P63 positive, and CK5/6 positive. In all positive cases the expression of staining was strong and diffuse, except for one case of adenocarcinoma in which CK20 was expressed focally and moderately. Of these tumors 34 were adenocarcinomas, 11 were squamous cell carcinomas, 18 stage I, 12 stage II and 15 stage III. Thirty‐five of the patients (35) received adjuvant chemotherapy. Platinum based chemotherapy was administrated (cisplatin) combined with gemcitabine. All patients received the same medication. For the cytological diagnosis of NSCLCs, the specimen from FNABs was stained using Papanicolaou and Giemsa stains. Immunocytochemistry was performed on air dried specimens using the alkaline phosphatase (APAAP) method. To estimate the ERCC1 expression the ERCC1 Ab‐2 (clone 8F1) IgG2b isotype mouse monoclonal antibody (Thermo Scientific, Fremont CA, USA) was used. To estimate the EGFR expression we used the monoclonal mouse anti human epidermal growth factor receptor clone H11, IgG1, Kappa isotype (DAKO, Carpinteria, CA, USA). We found that a dilution of 1:200 with an 1h incubation in a microwave oven 750W for 3 cycles was optimal, and a very light hematoxylin counterstain was performed before counting. For ERCC1, stromal cells surrounding the tumor areas served as internal positive controls and for EGFR we used tissue from breast cancer as positive control. For negative control we omitted the primary antibody.
The slides were examined using 10× ocular lens and 10 fields per slide (at least 1000 cells) were selected randomly and counted using an 40× objective lens.
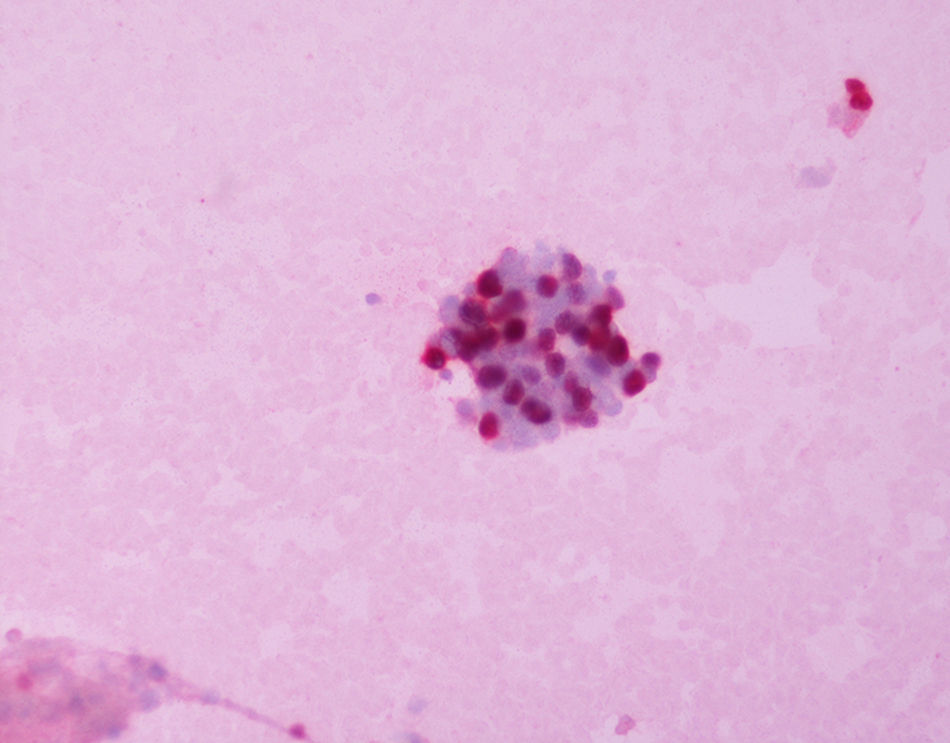
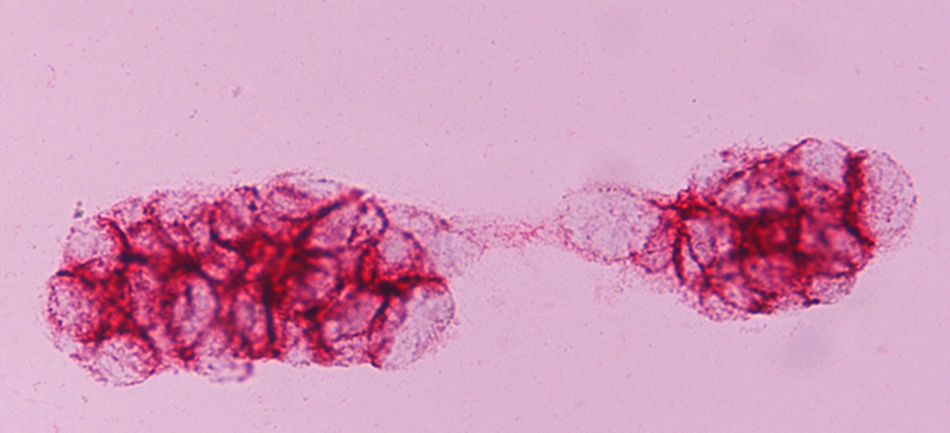
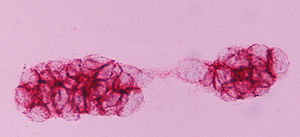
ERCC1 expression was nuclear (Fig. 1), and EGFR transmembrane (Fig. 2). The stain intensity was scored by two trained cytopathologists (AK and DT). Each cell was scored as 0, 1, 2, 3 which corresponded to negative, weak, moderate and strong staining intensities. Percentages of stained cells were counted and a final score was calculated by summing the products of staining intensities (0–3) and distributions (0–100%).The scores ranged from 0 to 300 (percentage of positive cells×staining intensity), and the median score was used as a cut‐off criterion. The scores 0, 1 were considered as negative and the scores 2 and 3 as positive. Data from this study were analyzed with the Pearson's chi‐square test. The level of statistical significance chosen was 0.05.
In cases positive for EGFR, mutational profile was not studied as this was beyond the purpose of our work.
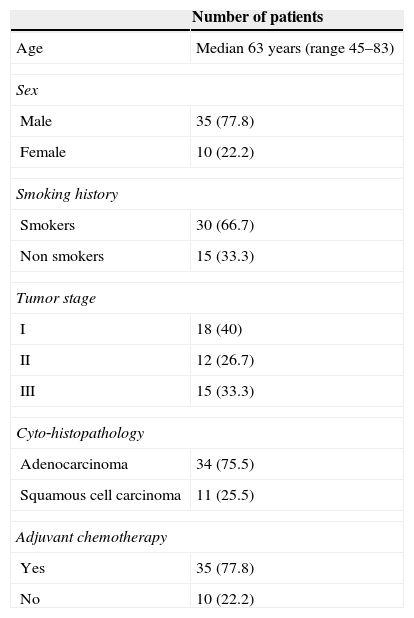
ResultsPatient characteristicsThis study included 45 patients (35 males and 10 females) with a median age of 63 years. Thirty (66.7%) had a history of smoking. Adenocarcinomas accounted for 75.5% and squamous cell carcinomas 25.5%. Stages I,II, and III were diagnosed in 40%, 26.7% and 33.3% respectively. Thirty five patients (77.8%) received adjuvant chemotherapy (Table 1) and the median survival of the patients was ranged between 2.9 and 9 years.
Characteristics of patients with NSCLC.
| Number of patients | |
|---|---|
| Age | Median 63 years (range 45–83) |
| Sex | |
| Male | 35 (77.8) |
| Female | 10 (22.2) |
| Smoking history | |
| Smokers | 30 (66.7) |
| Non smokers | 15 (33.3) |
| Tumor stage | |
| I | 18 (40) |
| II | 12 (26.7) |
| III | 15 (33.3) |
| Cyto‐histopathology | |
| Adenocarcinoma | 34 (75.5) |
| Squamous cell carcinoma | 11 (25.5) |
| Adjuvant chemotherapy | |
| Yes | 35 (77.8) |
| No | 10 (22.2) |
The expression of ERCC1 was evaluable in all 45 patients, and the median score was 10.
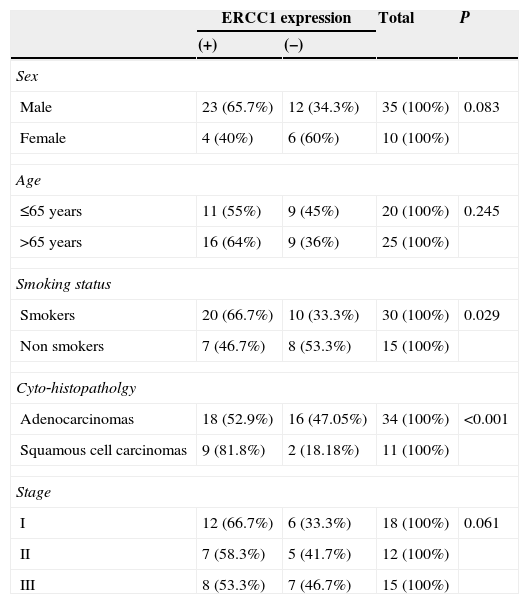
Patients were divided into 2 groups about this median value. 27 patients had ERCC‐1 positive tumors and a score ≥10 and 18 had ERCC‐1 negative tumors and a score of < 10. ERCC‐1 was frequent in squamous cell carcinoma (81.8% for squamous cell carcinomas versus 52.9% for adenocarcinomas, p<0.001) and in smokers (66.7% for smokers versus 46.7% for non smokers p=0.029) (Table 2).
Association between ERCC1 expression and clinical variables.
| ERCC1 expression | Total | P | ||
|---|---|---|---|---|
| (+) | (−) | |||
| Sex | ||||
| Male | 23 (65.7%) | 12 (34.3%) | 35 (100%) | 0.083 |
| Female | 4 (40%) | 6 (60%) | 10 (100%) | |
| Age | ||||
| ≤65 years | 11 (55%) | 9 (45%) | 20 (100%) | 0.245 |
| >65 years | 16 (64%) | 9 (36%) | 25 (100%) | |
| Smoking status | ||||
| Smokers | 20 (66.7%) | 10 (33.3%) | 30 (100%) | 0.029 |
| Non smokers | 7 (46.7%) | 8 (53.3%) | 15 (100%) | |
| Cyto‐histopatholgy | ||||
| Adenocarcinomas | 18 (52.9%) | 16 (47.05%) | 34 (100%) | <0.001 |
| Squamous cell carcinomas | 9 (81.8%) | 2 (18.18%) | 11 (100%) | |
| Stage | ||||
| I | 12 (66.7%) | 6 (33.3%) | 18 (100%) | 0.061 |
| II | 7 (58.3%) | 5 (41.7%) | 12 (100%) | |
| III | 8 (53.3%) | 7 (46.7%) | 15 (100%) | |
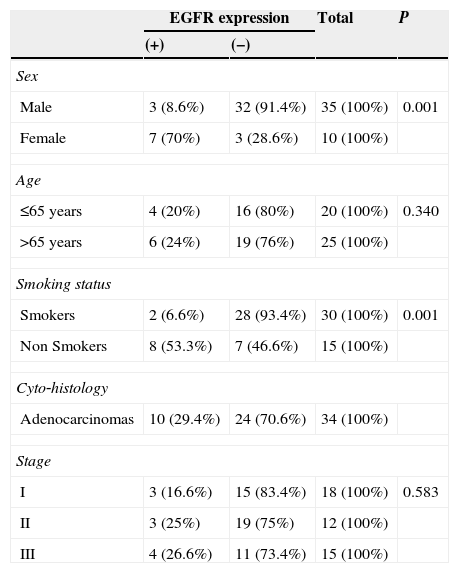
EGFR expression was found in 10 patients (22.2%). The frequency of EGFR expression was found to be significantly related to female gender (70% for females vs. 8.6% for males, p<0.001), smoking history (53.3% for non smokers vs. 6.6% for smokers, p<0.001). EGFR was expressed only in adenocarcinomas (in 10 of 34 tumors, 29.4%). No expression was found in squamous cell carcinomas (Table 3).
Association between EGFR expression and clinical variables.
| EGFR expression | Total | P | ||
|---|---|---|---|---|
| (+) | (−) | |||
| Sex | ||||
| Male | 3 (8.6%) | 32 (91.4%) | 35 (100%) | 0.001 |
| Female | 7 (70%) | 3 (28.6%) | 10 (100%) | |
| Age | ||||
| ≤65 years | 4 (20%) | 16 (80%) | 20 (100%) | 0.340 |
| >65 years | 6 (24%) | 19 (76%) | 25 (100%) | |
| Smoking status | ||||
| Smokers | 2 (6.6%) | 28 (93.4%) | 30 (100%) | 0.001 |
| Non Smokers | 8 (53.3%) | 7 (46.6%) | 15 (100%) | |
| Cyto‐histology | ||||
| Adenocarcinomas | 10 (29.4%) | 24 (70.6%) | 34 (100%) | |
| Stage | ||||
| I | 3 (16.6%) | 15 (83.4%) | 18 (100%) | 0.583 |
| II | 3 (25%) | 19 (75%) | 12 (100%) | |
| III | 4 (26.6%) | 11 (73.4%) | 15 (100%) | |
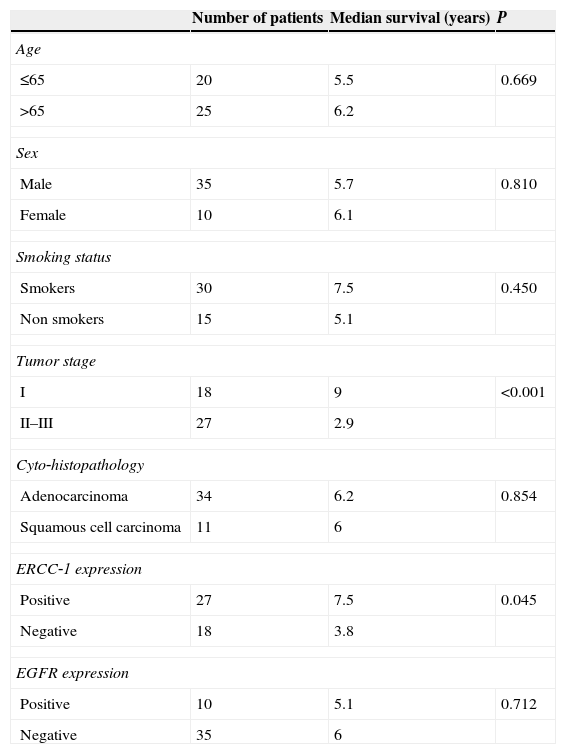
Patients with positive ERCC1 expression survived longer (median overall survival 7.5 years for ERCC1 positive vs. 3.8 years for ERCC‐1 negative, p=0.045). EGFR expression was not associated with survival (median overall survival 5.1 years for EGFR positive patients vs. 6 years for negative p<0.712). Median survival was found to be statistically significant with tumor stage (median survival 9 years in stage I tumors and 2.5 years in stage II and III p<0.001) (Table 4). The median survival was 6.2 years for adenocarcinomas and 6 years for squamous cell carcinomas (p=0.854) (Table 4).
Univariable analysis for factors related to overall survival.
| Number of patients | Median survival (years) | P | |
|---|---|---|---|
| Age | |||
| ≤65 | 20 | 5.5 | 0.669 |
| >65 | 25 | 6.2 | |
| Sex | |||
| Male | 35 | 5.7 | 0.810 |
| Female | 10 | 6.1 | |
| Smoking status | |||
| Smokers | 30 | 7.5 | 0.450 |
| Non smokers | 15 | 5.1 | |
| Tumor stage | |||
| I | 18 | 9 | <0.001 |
| II–III | 27 | 2.9 | |
| Cyto‐histopathology | |||
| Adenocarcinoma | 34 | 6.2 | 0.854 |
| Squamous cell carcinoma | 11 | 6 | |
| ERCC‐1 expression | |||
| Positive | 27 | 7.5 | 0.045 |
| Negative | 18 | 3.8 | |
| EGFR expression | |||
| Positive | 10 | 5.1 | 0.712 |
| Negative | 35 | 6 | |
No correlation between the expression of ERCC‐1 and the degree of tumor differentiation was made due to the fact that diagnosis was made from small tumor FNA samples with limited quantity of tissue, so not enough to make conclusions about differentiation of the whole tumor.
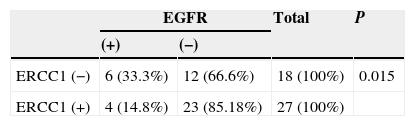
The relationship between ERCC1 and EGFR expression
Negative ERCC1 expression was significantly associated with the presence of EGFR expression. Of 18 patients with negative ERCC1 expression, 6 (33.3%) had an EGFR expression whereas only 4 patients (14.8%) out of 27 with positive ERCC1 expression had an EGFR expression (p=0.015) (Table 5).
DiscussionLung cancer continues to be the leading cause of cancer death worldwide. The prognosis of this disease remains poor, even in patients with NSCLCs treated by surgical resection, because undetectable but remaining viable tumor cells often cause local and distant recurrences after initial treatment. Postoperative chemotherapy has been attempted as part of standard therapy to improve the prognosis in patients undergoing surgery. DNA repair proteins, including ERCC‐1 have been studied11 as predictive11,12 factors for survival in patients with NSCLC.13–19 Epidermal growth factor receptor (EGFR) is a protein tyrosine kinase that is able to control intracellular pathways, and its activation promotes tumor proliferation, angiogenesis and metastasis.20 EGFR overexpression as detected by immunohistochemistry has been observed in at least two thirds of NSCLC, thus paving the way for monoclonal antibodies such as cetuximab, or more recently, panitumumab directed at the extracellular domain of EGFR, to prevent ligand binding and subsequent receptor activation or activate immune response against tumor cells.20
EGFR is commonly over expressed in NSCLC and the lack of consistent methods for evaluating its expression has led to inconclusive conclusions about EGFR as a prognostic factor.16
The present study was performed for first time in cytopathological specimens(FNABs) using immunocytochemistry, to determine if ERCC1 and EGFR expressions are prognostic markers for resected NSCLC in patients treated in a single institution. Our findings indicate that patients with ERCC‐1 positive tumors survived significantly longer after surgery than those with ERCC1 negative tumors (Table 4), which is in accordance with the findings of other recent studies11,14,18 but not in agreement with others12,13,15,16 that indicate a better outcome in patients with low expression levels of ERCC1.
ERCC1 expression was identified as positive (scores 2+ and 3+) in the majority of NSCLCs and seems to be an independent prognostic marker of longer survival. In addition EGFR expression was positive (scores 2+ and 3+) in the minority of NSCLCs and only in adenocarcinomas, more frequently in ERCC1‐negative (scores 0 and 1+) tumors, suggesting that it is not an independent prognostic marker for the outcome of the patients suffering from NSCLC.
In our study EGFR expression was more commonly found in tumors not expressing ERCC1 (Table 5). The relationship between EGFR mutations and ERCC1 expression has not been investigated in patients with NSCLC11 although there is a report which found no correlation between ERCC‐1 and EGFR mRNA expression.16 One possible explanation is that the pathogenesis of ERCC1 positive tumors differs from that of EGFR mutant tumors as depicted by the “oncogene addiction” theory. In this study ERCC1 expression was more frequent in squamous cell carcinomas and in smokers and EGFR expression only in adenocarcinomas and was more frequent in women and non smokers (Tables 2 and 3), this may be due to the infrequency of EGFR mutation in these patient groups.19 Simon et al. conclude that resected NSCLC patients with high ERCC‐1 expression (>50) have a better survival rate compared to patients with low ERCC‐1 expression (<50).21
Current data suggest that ERCC1 is a potentially useful marker for predicting clinical resistance to platinum compounds such as cisplatin, carboplatin and oxaliplatin. However, no study has yet provided sufficiently robust evidence to consider ERCC1 as a marker for resistance to all types of chemotherapy. Before the potential of ERCC1 as a predictor of chemosensitivity can be fully evaluated, several technical and practical obstacles should be addressed. First, the diagnosis is made from small tumor samples (biopsies) with limited quantity of bronchial tissue. Since ERCC1 immunostaining is characterized by heterogeneity, suboptimal sampling might mean that the biopsy is not necessarily representative of the whole tumor. Furthermore, tumor material could be obtained either from the primary or the metastatic sites. This may be significant since it has not been shown that ERCC1 expression is identical in the primary tumor and its metastatic locations. Secondly, studies associating ERCC1 with resistance to platinum compounds have so far been conducted mainly by DNA or RNA molecular analysis, which is both technically demanding and expensive. The IALT (International Adjuvant Lung Cancer Trial)‐bio study had the advantage of using immunohistochemistry to assess ERCC1 expression, which is a feasible technique in the everyday practice of a pathology laboratory. Should the technical aspects of ERCC1 immunohistochemistry become standardized and optimized, this methodology could rapidly be translated into a clinical reality after other confirmatory prospective studies. Currently, however, difficulties, including the identification of an optimal “cut‐off” value for ERCC1, the subjectivity of the pathological examination, particularly in prospective studies, and the heterogeneity of staining, that makes the technique of tissue micro‐arrays not really applicable, limit the clinical usefulness of such procedures. Other difficulties, such as different genetic backgrounds in the population, or interaction with smoking habits, should be further discussed. In any case, meticulous methodological considerations and standardization are mandatory. It is necessary that future larger and prospective studies should be extensively discussed within multidisciplinary groups including highly skilled pathologists and bio‐statisticians.22
In conclusion, we were able to use cytological specimens (FNABs) to study the expressions of ERCC1 and EGFR and their role in patients with NSCLC. ERCC1 expression as determined immunocytochemically, was identified as a prognostic marker of longer survival in NSCLCs. In addition, EGFR expression was more frequently found in ERCC‐1 negative tumors, but did not affect survival in the present study. Perhaps more studies for the status of the genes and further validation of the correlation between ERCC1 and EGFR expressions in longer number of patients are warranted.
National Comprehensive Cancer Network (NCCN) – The NCCN's clinical practice guidelines regarding NSCLC includes information about the role of ERCC1 as both a prognostic and predictive biomarker for NSCLC. However, the guidelines do not include recommendations about the use of ERCC1 testing in NSCLC patients.23
Test accuracy and reliability in measuring ERCC1 expression (analytic sensitivity and specificity).
ERCC1 testing is performed by IHC using an antibody to detect the ERCC1 protein. Two studies evaluated the specificity of the 8F1 ERCC1 antibody and yielded conflicting results: Niedernhofer and colleagues (2007) compared the performance of 8F1 with that of a second commercially available antibody, FL‐297. Test samples included normal human fibroblasts (positive control) and cells from patients with inherited sequence variants in the ERCC1 and xeroderma pigmentosum complementation group F (XPF) genes. Using the FL‐297 antibody, immunoblotting of cell lysates revealed a single band of the expected molecular weight in normal fibroblasts, but reduced levels of the protein in the patient cells. Using the 8F1 antibody, however, immunoblotting of cell lysates from normal fibroblasts revealed two separate bands: a band of unknown origin and a band corresponding to the ERCC1 protein. In the patient cell lines, only a single band was seen, which the authors state represented a protein of unknown origin that is cross‐reacting to the 8F1 antibody. In a second experiment, normal fibroblasts and cells from the patient with an XPF gene variant were differentially labeled and co‐cultured. Immunostaining revealed that the FL‐297 antibody was reactive in the normal fibroblasts, but not in the cells from the patient. In contrast, the 8F1 antibody yielded nuclear staining in all cells. Immunostaining of cells from the patient with an ERCC1 gene variant was not reported. The authors concluded that these results suggest that the 8F1 antibody is not specific for the ERCC1 protein and cannot discriminate between ERCC1‐positive and ERCC1‐deficient cells. Data confirming decreased expression of ERCC1 in the patient cell lines were not included in this study.24
Olaussen et al.25 further studied the specificity of the 8F1 antibody in response to the claim by Niedernhofer and colleagues (2007) that this antibody may not be specific for ERCC1. Several complementary approaches using small interfering RNAs (siRNAs) were utilized. First, siRNAs designed to block ERCC1 expression were used to deplete two epithelial carcinoma cell lines (including one NSCLC cell line) of ERCC1 protein. Using the 8F1 antibody, immunoblotting detected a single band of the expected molecular weight in each cell line. After treatment with the siRNA, this band disappeared. In a second experiment, cell pellets from treated and untreated cell lines were fixed and embedded using the same methods used for resected lung tumors. Subsequently, IHC was performed using the 8F1 antibody. Nuclear ERCC1 staining was clearly evident in the untreated control cells; however, this staining was absent in cells that were transfected with the siRNA. The authors concluded that these results suggest that 8F1 is specific when used for the immunohistochemical detection of ERCC1 in NSCLC samples.24,25
Two studies evaluated the importance of specimen type for ERCC1 expression testing: Taillade and colleagues26 evaluated the expression levels of ERCC1 (in addition to four other biomarkers for NSCLC) in both bronchial biopsies and surgical specimens (i.e., resected tumors) in 34 patients. The 8F1 antibody was used for IHC and two separate pathologists scored each sample blindly. Results were dichotomized into negative and positive based on a cutoff of 10% ERCC1 antibody reactivity within tumor cells (i.e., a positive result indicated that at least 10% of cells demonstrated a reactivity to the ERCC1 antibody). Seven samples were found to lack a valid internal control and were excluded from the analysis. The discordance in scoring between the two pathologists (indicated by a discrepancy >20% in staining percentage) was 7%. Overall, when comparing the results obtained from biopsies and surgical specimens, a statistically significant correlation was found (correlation coefficient r=0.83; P<0.0001). With the cutoff of 10%, 8 (30%) biopsies and 11 (41%) resected tumors were found to be positive for ERCC1. Thus, the discordance between specimen types, when examining positive versus negative results, was 9% (95% confidence interval [CI], 0% to 18.4%). The authors concluded the above results suggest that, while there is a strong correlation between ERCC1 levels in bronchial biopsies and resected tumor specimens, discordance is evident for different specimen types in some cases.26
Gomez‐Roca and colleagues27 assessed the levels of ERCC1 in primary NSCLC tumor specimens compared with the levels found in corresponding metastases. IHC using the 8F1 antibody was performed in 49 NSCLC patients who had both types of test specimens available. Using an H score (semiquantitative scores calculated by summing the products of staining intensities and distributions) of 1 to classify the ERCC1 expression, 18 primary tumors and 26 metastatic lesions were found to be ERCC1 positive. However, there was a significantly higher level of ERCC1 expression in metastases than in primary tumors (P=0.04). ERCC1 expression was at least five times greater in metastatic lesions (especially brain metastases) than in corresponding tumors. ERCC1 status was discordant in 20 of the 49 (41%) patients. The authors concluded that these results suggest that it may be appropriate to examine ERCC1 levels in both primary tumors and metastatic lesions prior to deciding on a treatment regimen.27
The body of evidence surrounding ERCC1 testing in NSCLC indicates that the analytical validity of ERCC1 testing using IHC may have limitations. Confirmatory prospective studies, with several centers and countries will be needed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors have no conflict of interests to declare.