Some conflicting results have been published about the relationship between TNF-α-308 gene polymorphism and chronic obstructive pulmonary disease (COPD). The aim of this study was to determine whether TNF-α-308 gene polymorphism was associated with smoking-related COPD and whether it was associated with pulmonary function parameters (PFTs), body mass index (BMI), and prognosis.
MethodsWe studied the frequencies of TNF-α-308 gene polymorphism in 90 male subjects (60 subjects with COPD and 30 healthy smokers) in a Caucasian population.
ResultsThere was no significant difference in the frequency of G/G and G/A gene polymorphisms in the COPD group compared with control subjects (p>0.05). We compared COPD patients as G/A gene polymorphism and G/G gene polymorphism; the PFTs and BMI before and after one year were not statistically significant (p>0.05). Also, the exacerbation and hospitalization data of COPD patients were not significant between these groups.
ConclusionIn conclusion, there was no difference between smoking-related COPD and the control group according to TNF α-308 gene polymorphism in a Caucasian population. In addition, it was shown that important determinants of prognosis of COPD such as FEV1, BMI, COPD exacerbation and hospitalization were not associated with TNF-α-308 gene polymorphism.
Foram publicados alguns resultados contraditórios sobre a relação entre o polimorfismo do gene TNF-α -308 e a doença pulmonar obstrutiva crónica (DPOC). O objectivo deste estudo foi determinar se o polimorfismo do gene TNF-α -308 estava associado à DPOC ligada ao tabagismo e se foi associado aos parâmetros de função pulmonar (PFTs), índice de massa corporal (IMC), e prognóstico.
MétodosEstudámos as frequências do polimorfismo do gene TNF-α -308 em 90 indivíduos do sexo masculino (60 indivíduos com DPOC e 30 fumadores saudáveis) numa população caucasiana.
ResultadosNão houve uma diferença significativa na frequência de polimorfismos genéticos G/G e G/A no grupo de DPOC, em comparação com o grupo de controlo (p>0,05). Comparámos os doentes com DPOC como polimorfismo genético G/A e polimorfismo genético G/G; os PFTs (parâmetros de função pulmonar) e o IMC (índice de massa corporal) antes e depois de um ano não eram estatisticamente significativos (p>0,05). Da mesma forma, os dados de agravamento e hospitalização dos doentes com DPOC não eram significativos entre estes grupos.
ConclusãoEm conclusão, não existiu uma diferença entre o grupo com DPOC ligada ao tabagismo e o grupo de controlo, de acordo com o polimorfismo do gene TNF α -308, numa população caucasiana. Além disso, foi mostrado que determinantes importantes do prognóstico da DPOC, tal como VEMS, IMC, exacerbações da DPOC e hospitalização não estavam associados ao polimorfismo do gene TNF-α -308.
Cigarette smoking is the most common and important cause of chronic obstructive pulmonary disease (COPD). However, COPD develops in only 10–15% of smokers.1 In addition, a familial predisposition for COPD and lung function is well known.2,3 These findings suggest that genetic factors are responsible for COPD. Alpha-1 antitrypsin deficiency is the best-known genetic risk factor for COPD, but such cases are estimated to account for only 1–2%.4
Tumor necrosis factor (TNF)-α is a cytokine primarily derived from macrophages. Increased concentrations of TNF-α have been found in induced sputum, bronchoalveolar lavage fluid, and bronchial biopsies from patients with COPD compared with healthy smokers.5–7 These findings have led researchers to further work on TNF-α. A genomic polymorphism resulting in the substitution of the nucleotide adenine (A) for guanine (G) at position-308 within a regulatory region of the TNF-α locus was discovered in 1992.8 This polymorphism at position-308 of the TNF-α gene showed elevated TNF-α secretion and blood levels in some studies.9,10
It is necessary to clarify the effects of genetic polymorphisms of the TNF-α gene promoter region for the development of COPD and disease progression. Some conflicting results have been published about the relationship between TNF-α-308 gene polymorphism and COPD. A meta-analysis of twenty four studies showed that TNF-α-308 gene polymorphism is associated with an increased risk of COPD in the Asian population, but this relationship is not significant in the Caucasian population.11 Although previous reports showed that the FEV1 and BMI values were lower in COPD patients than in smoker controls, there was no significant difference among G/G, G/A and A/A gene polymorphisms in COPD patients.12–14 In a report that evaluates the prognosis of COPD and TNF-α gene polymorphism, COPD exacerbation was found to be the most common cause of death.12 The aims of the present study were to determine whether TNF-α-308 gene polymorphism was associated with smoking-related COPD and whether it was associated with pulmonary function parameters (PFTs), body mass index (BMI), and prognosis in a Caucasian population.
MethodsSubjectsThe study was approved by the Research Ethics Committee of Erciyes University School of Medicine in Kayseri, Turkey and all subjects gave written informed consent. Ninety subjects were enrolled from August 2010 to December 2011. The patient group was recruited from the Pulmonary Outpatient Department. It consisted of 60 male adults with COPD. The diagnosis of COPD was made according to the Global Initiative for Chronic Obstructive Lung Disease standards.15 The criteria of enrollment were as follows: (1) >40 years old, (2) a smoking history of 20–80 pack-years, (3) available spirometry data for the previous year, (4) post-bronchodilator FEV1/FVC <70%, and (5) patient's consent. Patients were excluded if they had had an exacerbation of COPD within the previous 6 weeks and significant reversibility (FEV1 or FVC>12% baseline and/or 200ml) to 2.5mg nebulized salbutamol.
The previous year's PFTs and BMI (calculated as weight in kg divided by the square of height in meters) were recorded from files. The annual number of hospitalizations and COPD exacerbations were examined during the last year. Exacerbation of COPD was defined as “an event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough and/or sputum that is beyond normal day-to-day variations, is acute in onset and may warrant a change in regular medication”.15 New PFTs were performed by experienced technicians and BMI was calculated.
The control group consisted of 30 male chronic heavy smokers with normal lung function who were recruited randomly from the smoking cessation clinic. The inclusion criteria were as follows: (1) male older than 40 years of age, (2) heavy smoker with a smoking history of 20–80 pack-years, (3) post-bronchodilator FEV1/FVC ratio>70%, FEV1% predicted>80%, and FVC % predicted>80%, (4) no other pulmonary disease.
SpirometerAll PFTs were performed by experienced lung function technicians with the same standard spirometer (Vmax20 system; SensorMedics, Yorba Linda, CA, USA) according to American Thoracic Society (ATS) recommendations.16 The best FVC measurement was recorded along with FEV1 and the FEV1/FVC calculations.
Genotyping protocolThree milliliters of whole peripheral blood was obtained from each study participant by phlebotomy into a tube containing ethylene diamine tetra-acetic acid (EDTA). DNA was extracted promptly using a MagNA Pure LC DNA Isolation Kit I (Cat. No. 03003990001, Ver. 17.0) supplied by Roche (Mannheim, Germany).
TNF alpha-308 promoter gene polymorphism (rs1800629) was genotyped using the LightCycler real-time PCR system. The primers and hybridization probe for genotyping the -308 promoter polymorphism were designed by and obtained from Tib MolBiol GmbH, Berlin, Germany. The PCR reaction was carried out using the LightCycler real-time PCR machine 480 II (Roche Diagnostics GmbH, Mannheim, Germany), with software version 1.5.0. A reaction volume of 20μl (with 2μl of DNA Master Mix, 1μl of primers probe, 1.6μl of 25mM MgCl2, 10.4μl H2O and 5μl genomic DNA (10ng)) was amplified in 96-well plates using the FastStart LC 480 II genotyping master kit (Roche Diagnostics GmbH). The PCR protocol for -308 promoter polymorphism consisted of initial denaturation at 95°C for 10min, followed by 45 cycles of denaturation (95°C for 10s, 4.4°C/s), annealing (60°C for 10s, 2.2°C/s) and elongation (72°C for 10s, 4.4°C/s). This was followed by melting curve analysis consisting of 1 cycle at 95°C for 30s (4.4°C/s), 40°C for 2min (1.5°C/s) and a temperature rise to 75°C at a slope of 0.19°C/s with continuous measurement of fluorescence.
Statistical analysisSPSS software (Statistical Package for the Social Sciences, version 15.0, SSPS Inc, Chicago, IL, USA) was used for statistical analyses. The distribution of continuous variables for normality was tested with the One-Sample Kolmogorov–Smirnov test and data are presented as mean±standard deviation (S.D.) or median and interquartile ranges, as appropriate. Demographic characteristics and parameters were compared using the Student t-test or Mann–Whitney U test for the continuous variables, where appropriate. Categorical variables were analyzed by the χ2-test. According to groups, the before and after values of the quantitative variables were assessed by the mean paired-sample t-test. The power of the study based on the gene polymorphism was 0.068 for (with alpha error of 0.05). Differences were considered statistically significant when p<0.05.
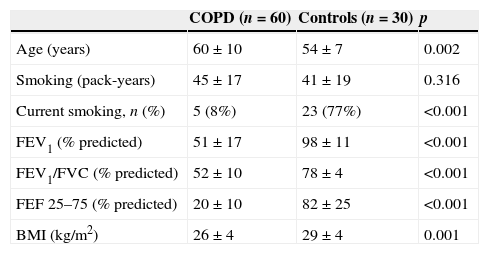
ResultsComparison of COPD group and control groupThe characteristics of the COPD (n=60) and the control groups (n=30) are summarized in Table 1. The COPD patients were older (60±10 vs. 54±7 years, p<0.05) than the control group but had a similar pack-year history (45±17 vs. 41±19 pack-years, p>0.05). PFTs (FEV1%, FEV1/FVC, FEF 25–75%) in the control group were near normal and significantly greater than those of the COPD group (p<0.001). Mean BMI was also significantly lower in the COPD group (26±4 vs. 29±4kg/m2, p=0.001).
Demographic characteristics and pulmonary test parameters of the COPD patients and healthy control groups.
| COPD (n=60) | Controls (n=30) | p | |
|---|---|---|---|
| Age (years) | 60±10 | 54±7 | 0.002 |
| Smoking (pack-years) | 45±17 | 41±19 | 0.316 |
| Current smoking, n (%) | 5 (8%) | 23 (77%) | <0.001 |
| FEV1 (% predicted) | 51±17 | 98±11 | <0.001 |
| FEV1/FVC (% predicted) | 52±10 | 78±4 | <0.001 |
| FEF 25–75 (% predicted) | 20±10 | 82±25 | <0.001 |
| BMI (kg/m2) | 26±4 | 29±4 | 0.001 |
Values are expressed as mean±SD or as absolute numbers. COPD=Chronic obstructive pulmonary disease, FEV1=Forced expiratory volume in 1s, FVC=Forced vital capacity, FEF25–75%=Forced expiratory flow 25–75%, BMI=Body mass index.
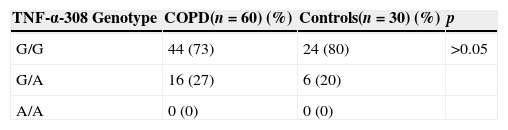
The genotype frequencies of TNF-α-308 gene polymorphisms in the COPD group were compared with those in control subjects. Forty-four (73%) patients had G/G and 16 (27%) patients had G/A gene polymorphisms in the COPD population; 24 (80%) patients had G/G and 6 (20%) patients had G/A gene polymorphisms in the control group. AA genotypes were not determined in any patient in the two groups. There was no significant difference in the frequency of G/G and G/A genotypes in the COPD population compared with control subjects (p>0.05) (Table 2).
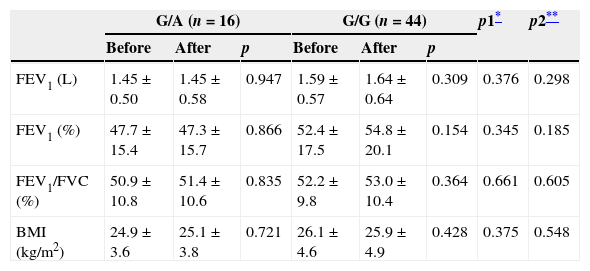
Comparison of COPD patients grouped by genotypeIn COPD patients, the mean age and smoking status of patients were compared as groups of TNF-α-308 G/A gene polymorphism (n=16) and G/G gene polymorphism (n=44). The mean age [61±11 (G/A)/60±10 years (G/G)], the amount of smoking [45±17 (G/A)/45±17 pack-years (G/G)] and active smoking [0 (G/A)/5 (G/G)] was not statistically significant in these groups (p>0.05). Also, a comparison of PFTs and BMI before and after one year were not statistically significant (p>0.05). Table 3 shows the comparison of PFTs and BMI in both groups.
Comparison of PFTs and BMI before and after one year grouped by gene polymorphism in patients with COPD.
| G/A (n=16) | G/G (n=44) | p1* | p2** | |||||
|---|---|---|---|---|---|---|---|---|
| Before | After | p | Before | After | p | |||
| FEV1 (L) | 1.45±0.50 | 1.45±0.58 | 0.947 | 1.59±0.57 | 1.64±0.64 | 0.309 | 0.376 | 0.298 |
| FEV1 (%) | 47.7±15.4 | 47.3±15.7 | 0.866 | 52.4±17.5 | 54.8±20.1 | 0.154 | 0.345 | 0.185 |
| FEV1/FVC (%) | 50.9±10.8 | 51.4±10.6 | 0.835 | 52.2±9.8 | 53.0±10.4 | 0.364 | 0.661 | 0.605 |
| BMI (kg/m2) | 24.9±3.6 | 25.1±3.8 | 0.721 | 26.1±4.6 | 25.9±4.9 | 0.428 | 0.375 | 0.548 |
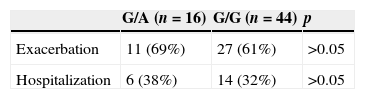
The exacerbation and hospitalization data of COPD patients are summarized in Table 4. Eleven (69%) patients who had G/A gene polymorphism and 27 (61%) patients who had G/G gene polymorphism in the COPD group had acute COPD exacerbation in the past year. Six (38%) patients with G/A gene polymorphism and 14 (32%) patients with G/G gene polymorphism were hospitalized because of COPD exacerbation. Neither of these differences were statistically significant (p>0.05).
DiscussionThe present study demonstrates that there is no difference in the frequency of G/A and G/G gene polymorphism in patients with COPD compared to healthy, smoking control subjects in a Caucasian population. Many studies have shown an association between COPD and TNF-α-308 gene polymorphism in an Asian population.17–20 However, in a Caucasian population, although studies have been conducted in different countries, most of them did not show any association; this was similar to our results.12,21–30 A large family study from a Boston early-onset COPD study and another American study are the only two studies to show an association between COPD and TNF-α-308 polymorphism in a Caucasian population.13,31 In addition, a study from our country recently showed a significant difference among COPD stages in terms of TNF-α 308 G/A polymorphism.32 However, a meta-analysis of data for 6118 subjects also showed that TNF-308 gene polymorphisms were significantly associated with an increased risk of COPD among an Asian population but not among a Caucasian population.11 In this situation, it seems that polymorphisms in the TNF-α-308 gene that stimulate the production of cytokines are not major genetic determinants for the development of COPD in most of the Caucasian population.
The major etiologic factor for the development of COPD is cigarette smoking. The risk of COPD development among people with a similar history of smoking may be different due to genetic predisposition and/or lifestyles. In a study investigating the association between antioxidant genes and COPD, the definition of “resistant smoker” was used for smokers who did not develop COPD.33 Therefore, we identified individuals with a smoking history of 20–80 pack-years and created similar patient and control groups. TNF-α-308 gene polymorphism was found to be ineffective in the development of COPD in these groups which had a similar smoking history.
The second major finding in the present study is that COPD patients with G/A and G/G gene polymorphism had similar PFTs (FEV1, FEV1%, FEV1/FVC) before and after one year evaluation. The FEV1 value was the most commonly used parameter for monitoring disease progression.15 Only one report exists that evaluates the prognosis of TNF-α-308 gene polymorphism in COPD patients.12 Keatings et al. showed that AA homozygous patients had less reversible airflow obstruction and a significantly greater mortality (both all-cause and respiratory deaths) on follow-up despite a shorter cigarette smoking history. In another study, the homogenous A genotype in COPD patients was accompanied by a significant reduction in the mean values of the post-bronchodilator FEV1, FEV1/FVC and FEF25/75% of predicted compared to heterozygous G/A genotypes in COPD.30 However, in the present study, there were no patients with A/A polymorphism for comparison purposes.
Another parameter that was shown to have prognostic importance in patients with COPD was BMI.34 In a study by Wilson et al., BMI was shown to be an important prognostic factor after FEV1 in male patients with COPD.35 Furthermore, low BMI was shown to be independently associated with reduced survival in patients with severe COPD.36 In the present study, BMI was significantly lower in patients with COPD than in the healthy smoker control group. When we examined the effect of the TNF-α-308 gene on BMI, neither the current BMI nor the BMI of the previous year were statistically significant between the patients with G/A and G/G gene polymorphisms.
The most important limitation of this study was the low number of patients. In addition, mortality was not examined in the present study. No patients had A/A gene polymorphism, so we have no data about A/A gene polymorphism in our population.
ConclusionIn conclusion, there was no difference between smoking-related COPD and control group according to TNF α-308 gene polymorphism in a Caucasian population. In addition, it was shown that important determinants of prognosis of COPD such as FEV1, BMI, COPD exacerbation and hospitalization were not associated with TNF-α-308 gene polymorphism.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors would like to thank Ferhan Elmalı for data analysis.