To the Editor,
The aim of lung transplant (LT) is not only to extend the survival rate of recipients but also to improve their quality of life (QoL).1 Several studies have been conducted to compare QoL before and after LT.2, 3, 4 However, fewer reports evaluated the long term QoL, specifically the period when some late complications tend to appear.5 These include chronic allograft rejection or bronchiolitis obliterans syndrome (BOS), which are the major cause for decreased patient life expectancy.5 In order to prevent or stabilize these complications, LT recipients maintain a high level of immunosuppressants for life. In turn, the immunosuppressive drugs induce different disorders, like arterial hypertension, chronic kidney failure, diabetes, hyperlipidemia, osteoporosis, and infections (which are the second cause of mortality after BOS), and increase the risk of malignancies, mainly skin cancers, post-transplant lymphoproliferative disorders and Kaposi's sarcoma.6
The authors here describe the data about complications related to LT and QoL of patients followed in LT outpatient clinic of Centro Hospitalar São João. Of the 83 patients, 37 (30 LT recipients and 7 LT candidates) completed the Medical Outcomes Study Short Form-36 (MOS SF-36), the London Chest Activity of Daily Living (LCADL) questionnaire and the Hospital Anxiety and Depression Scale (HADS). The lung transplant recipients (n = 30) were grouped according to the time of transplant [<1.5 years post-LT (n = 5); 1.5–3 years post-LT (n = 6); >3 years post-LT (n = 19)]. The parameters assessed in the questionnaires were compared between the groups pre- and post-transplant.
Among all patients, COPD (27%), silicosis (19%), hypersensitivity pneumonitis (19%) and alfa-1 antitrypsin deficiency (16%) were the most common underlying diagnoses. Of the 30 LT recipients, the most frequent complications were: osteoporosis (46.7%), CMV infection (40%), renal dysfunction (33%), hyperlipidemia (33%), arterial hypertension (30%), and acute rejection (30%); only one patient had BOS. We observed a positive correlation between the complications frequency and the physical limitation in the self-care domain of LCADL (r = 0.45; p = 0.01).
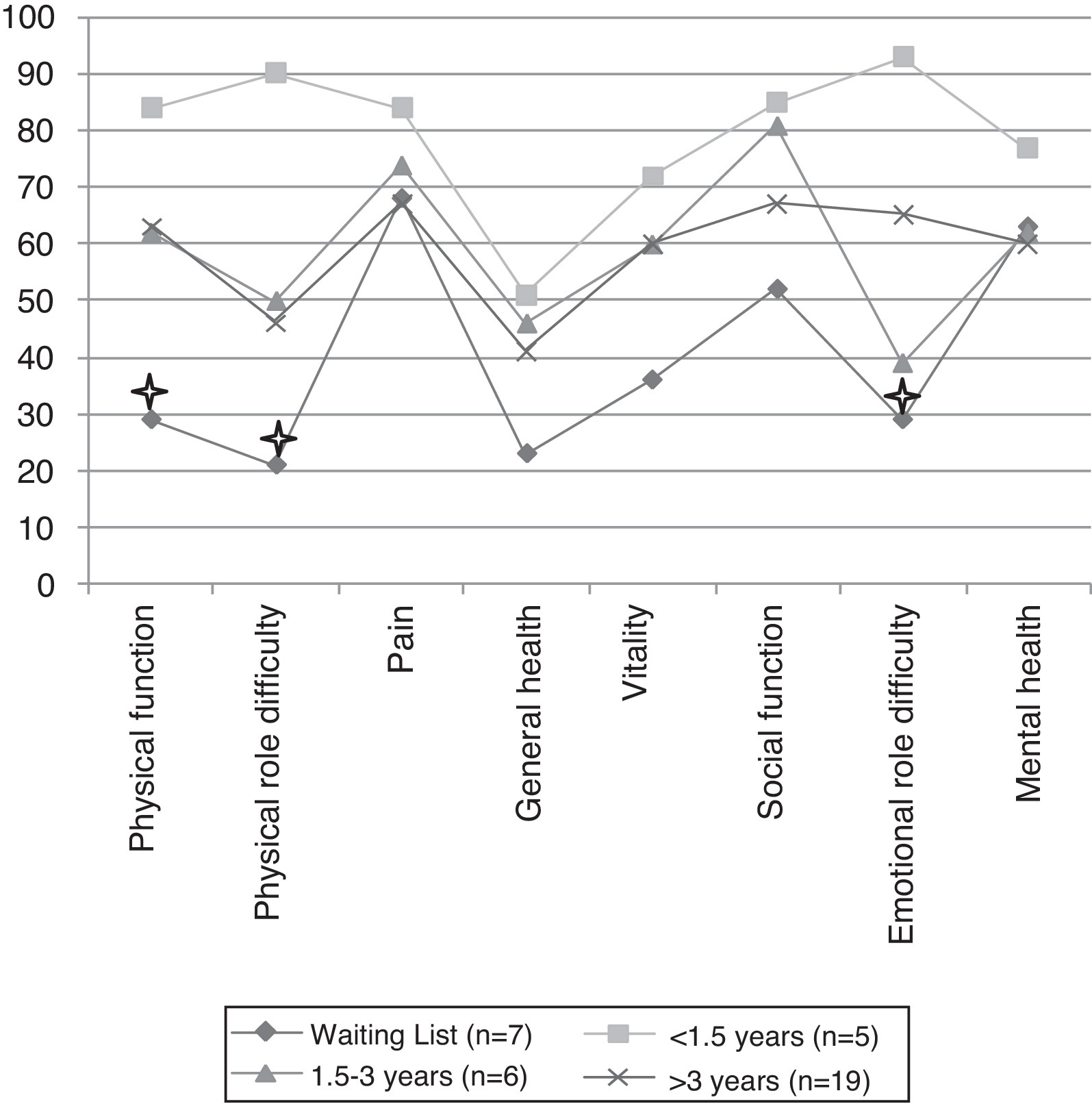
Compared with candidates, recipients had better QoL assessed by the MOS SF-36 (Figure 1). However, only the patients with less than 1.5 years of transplant reported statistically significant differences in QoL relative to candidates, particularly in the physical function dimension (84.0 ± 17.1% vs. 29.3 ± 22.4%, p = 0.01), physical role difficulty dimension (90.0 ± 22.3% vs. 21.4 ± 39.3%, p = 0.04) and emotional role difficulty dimension (93.4 ± 14.7 vs. 28.6 ± 40.5%, p = 0.04). No statistically significant difference was found among recipients grouped by time since their transplant, but there is a downward trend in QoL over the years (Figure 1).
) = p < 0.05 in comparison between candidates and patients with <1.5 years after lung transplant." />Figure 1. Quality of life of candidates and lung transplant recipients. Asterisk (
) = p < 0.05 in comparison between candidates and patients with <1.5 years after lung transplant.Levels of depression are significantly lower in transplant recipients than in candidates (moderate–severe depression: 12% in recipients vs. 100% in candidates, p < 0.001), with no differences among the three groups after transplantation. No statistically significant difference was found in levels of anxiety.
Therefore, the improvement of the QoL of LT recipients is highest early after transplantation, with a slight but not significant decrease over the years. This may reflect the accumulation of complications with time after LT, which affect physical activity.
This analysis had two essential limitations: (1) the small sample size and (2) it is not a longitudinal pre- and post-transplant evaluation of patients QoL.
Santana, et al. described the effects of QoL measures in the management of LT patients, namely in lung allocation decisions, and advised their use in clinical routine.3 In addition, improving QoL appears as the primary goal of the LT for diseases such COPD (the major common indication to LT), where a survival advantage has not yet been proven.7 Therefore, we highlight the importance of including QoL measures in assessing the outcomes in LT.
Conflicts of interestThe authors have no conflicts of interest to declare.
Corresponding author. car_veronica@sapo.pt