This study evaluated the protective antioxidant effect of melatonin on lung injury as a remote organ after skeletal muscle ischemia–reperfusion in rats.
MethodsThirty male Wistar rats were allocated randomly into three experimental groups: operated with no ischemia (Sham) group, ischemia–reperfusion group and ischemia–reperfusion + melatonin group. Hind limb ischemia was induced by clamping the femoral artery. After 2 h ischemia, the clamp was removed and the animal underwent 24 h reperfusion. Rats in the ischemia–reperfusion + melatonin group received melatonin (10 mg/kg i.v.), immediately before the clamp was removed. At the end of the trial, animals were euthanized and the lungs were removed for water content determination, histopathological and biochemical studies.
ResultsIn the ischemia–reperfusion + melatonin group, tissues showed less intense histological abnormalities such as neutrophilic infiltration, intra-alveolar hemorrhage and edema compared with the ischemia–reperfusion group. Histopathologically, there was a significant difference (P < 0.05) between the two groups. The lung water content in the ischemia–reperfusion + melatonin group was significantly lower than the ischemia–reperfusion group (P < 0.05). Lung tissue myeloperoxidase (MPO) activity and nitric oxide (NO) level were significantly (P < 0.05) increased by ischemia–reperfusion. The increase in these parameters was reduced by melatonin.
Comparing the ischemia–reperfusion + melatonin group with the sham group, no significant increase in all analyzed aspects of the research was observed.
ConclusionsThese findings suggest that melatonin has preventive effects in lung tissue injury after transient femoral artery occlusion.
The pathophysiology of ischemia–reperfusion injury has been previously described. Polymorphonuclear leukocytes and free radical species have been shown to have pivotal roles in the etiology.1 Skeletal muscle ischemia–reperfusion resulting from trauma, limb revascularization, orthopedic surgery and free-flap reconstruction or any other condition not only leads to local damage, but also causes severe injury involving destruction of remote organs.2, 3, 4Many studies have shown that several agents such as caffeic acid phenethyl ester or erdosteine,5, 6 N-acetylcysteine,4 zinc aspartate,7 trapidil8 and tramadol9 are useful against lung injury induced by oxidative stress damage in ischemia–reperfusion.
Melatonin, the chief hormone of the pineal gland, is a well-known potent antioxidant and free radical scavenger10, 11 that can counteract the damaging effects of free radicals.12, 13 Melatonin also shows a protective effect on lung injuries induced by ischemia–reperfusion.14 There have been no studies to test the effect of melatonin on lung histological changes induced by skeletal muscle ischemia–reperfusion. The aim of this study was to examine the effects of melatonin on acute lung injury in a rat model of skeletal muscle ischemia–reperfusion by transient femoral artery occlusion.
Materials and methodsAll experimental procedures were performed according to the guidelines for the ethical treatment of experimental animals and approved by Islamic Azad University School of Veterinary Science, Animal Care and Use Local Ethics Committee.
Experimental groupsThirty male Wistar rats weighing 300 ± 35 g were used in this study. They were maintained under constant room temperature of 25 ± 2 °C, relative humidity of 75 ± 5%, 12 h/12 h light/dark cycle, with ad libitum access to water and commercial food and were placed in individual cages. Animals were randomly allocated into three experimental groups of ten rats each:
Group I – Sham group with no ischemia–reperfusion. The animals were subjected to all operative procedures, except arterial occlusion and reperfusion. After isolation and exposure of the femoral artery for 2 h, the animals received 2 ml of 0.9% saline via the external jugular vein.
Group II (ischemia–reperfusion group) – The animals were subjected to 2 h of ischemia followed by 24 h of reperfusion. Two milliliters of 0.9% saline was administered intravenously prior to reperfusion.
Group III (ischemia–reperfusion + melatonin group) – All animals underwent 2 hours of ischemia by occlusion of the femoral artery followed by 24 h of reperfusion. A solution of 10 mg/kg melatonin was administered immediately via the external jugular vein, with a total volume of 2 ml.
SurgeryAnesthesia was induced using ketamine plus xylazine (10 mg/kg and 50 mg/kg i.m., respectively). After induction of anesthesia, the left hind limb was prepared for sterile surgery. A skin incision was made on the medial surface of the left hind limb and the femoral artery was isolated and clamped with a vascular forceps for 2 h. Following the ischemic period, the vascular forceps was removed and then the surgical site was routinely closed. Rats were returned to their cages with a commercial balanced diet and water ad libitum.
Specimen collectionAt the end of the trial, the rats were euthanized with an overdose of intraperitoneal pentobarbital injection (300 mg/kg) and the lungs were removed en bloc. The right lungs were used for water content determination and histopathological analysis under light microscope and the left lungs were stored at −20 °C till the biochemical analysis. The lung tissue homogenate and supernatant samples were prepared as described by Yildirim et al.15
Biochemical assayMyeloperoxidase (MPO) activity and nitric oxide (NO) levels were studied in lung tissues. The activity of MPO was analyzed spectrophotometrically as described by Wei et al.16 NO level in lung tissue was measured by Griess reaction.17
Lung wet/dry weight ratioLung wet/dry weight ratio was used as an indicator of pulmonary edema. The lower lobe of the right lung from each animal was weighed and placed in a vacuum oven (at 54 °C) until a stable,18 dry weight was achieved. The ratio of lung wet weight to dry weight was then calculated.
Histopathological examinationThe anterior lobe of the right lung (tissue) was placed in a 10% formalin solution and processed routinely by embedding in paraffin and then tissues were sectioned into 5 μm pieces and stained with hematoxylin & eosin stain. Pulmonary injury was graded into four grades as follows: grade 0, no diagnostic change; grade 1, mild neutrophil leukocyte infiltration and mild to moderate interstitial congestion; grade 2, moderate neutrophil leukocyte infiltration, perivascular edema formation, and partial destruction of pulmonary architecture; and grade 3, dense neutrophil leukocyte infiltration and complete destruction of pulmonary structure.19 A total of five slides from each lung sample were randomly screened, and the mean was accepted as the representative value of the sample.
The Statistical Package for the Social Sciences for Windows 11.5 was used for statistical analysis. All values were given as mean ± standard deviation. Differences between groups were statistically analyzed by Kruskal–Wallis test (ANOVA) and chi-square test. Statistical significance was accepted as P < 0.05.
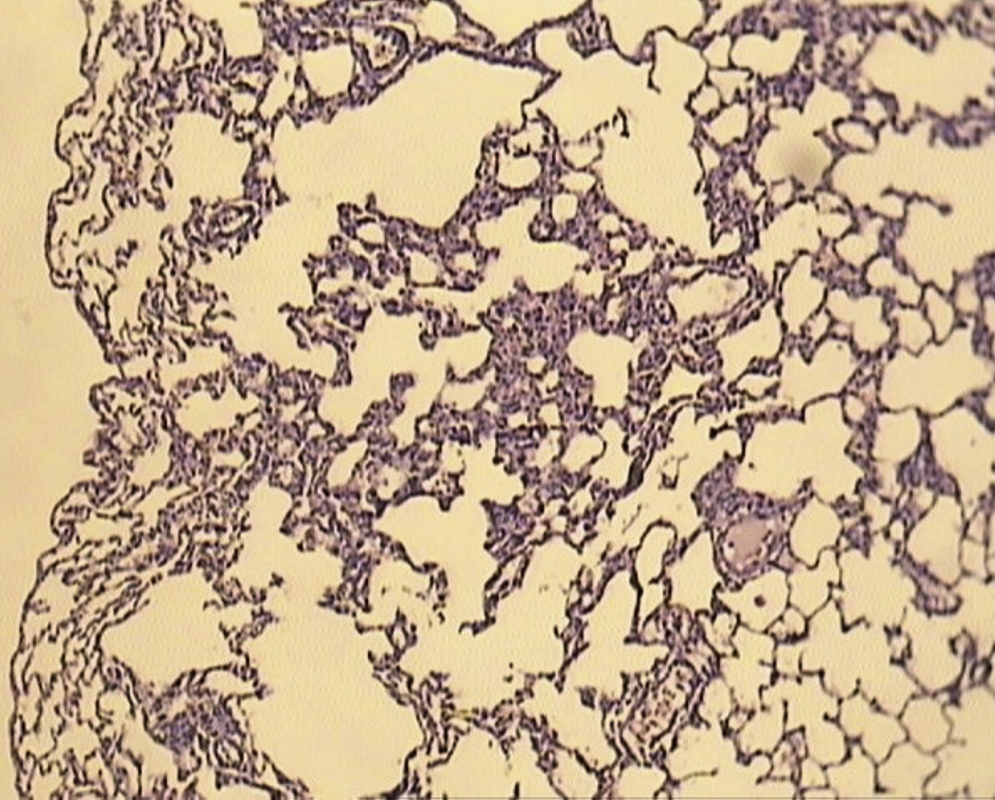
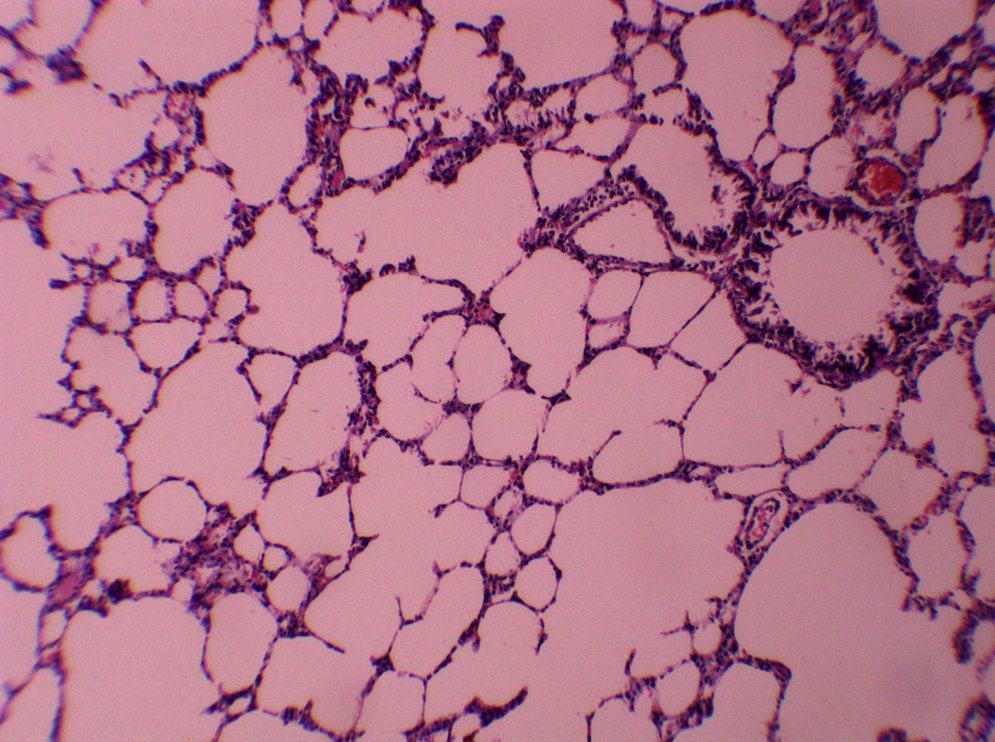
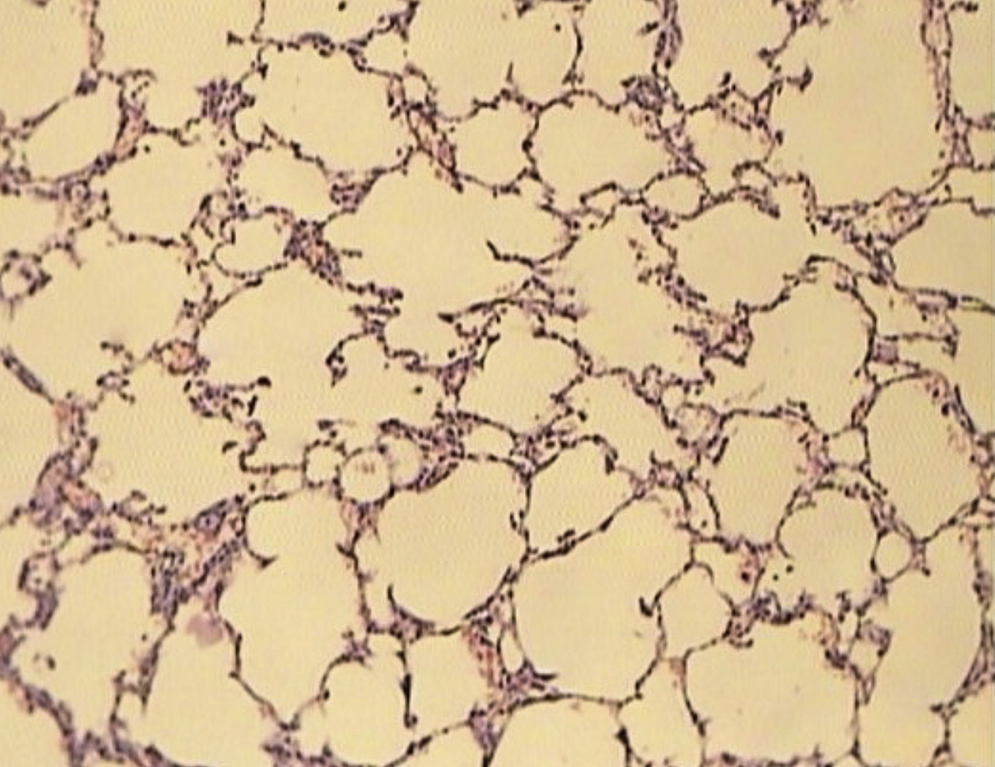
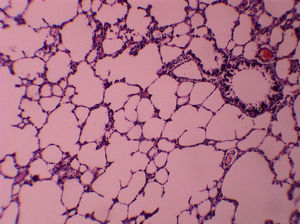
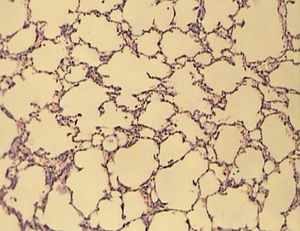
ResultsAll of the rats survived until the end of the study period. Data belonging to histological changes from lung samples after reperfusion are represented in Table 1. In the ischemia–reperfusion group, tissues showed histological changes with partial destruction of pulmonary architecture, neutrophilic infiltration, intra-alveolar hemorrhage and edema (Figure 1). Grades 1, 2 and 3 of lesions were obtained in the ischemia–reperfusion group, with an average of 2.3. These pathological changes in the ischemia–reperfusion + melatonin group, particularly neutrophilic infiltration and edema, were much less than in the ischemia–reperfusion group (Figure 2). Four animals in the ischemia–reperfusion + melatonin group presented no injury and the others had lesions grades 1 and 2, averaging 0.7. Histopathologically, there was a significant difference (P = 0.001) between the two groups. The average of histological changes in the sham group (0.2) was lower than the ischemia–reperfusion + melatonin group, but with no statistical significance (Figure 3).
Table 1. Histopathologic evaluation, myeloperoxidase (MPO) activity and level of nitric oxide (NO) in lung tissue for each group.
| Groups | Histological score | MPO (U/g protein) | NO (μmol/g protein) | Lung wet/dry weight |
| Sham | 0.2 ± 0.46 | 0.06 ± 0.04 | 20.18 ± 1.06 | 4.10 ± 0.21 |
| Ischemia–reperfusion | 2.3 ± 0.51 * | 0.47 ± 0.11 * | 58.07 ± 11.45 * | 8.12 ± 1.37 * |
| Ischemia–reperfusion + melatonin | 0.7 ± 0.17 + | 0.17 ± 0.03 + | 25.68 ± 4.30 + | 5.01 ± 0.39 + |
+ P > 0.05 versus sham group, P < 0.05 versus ischemia–reperfusion group.
* P < 0.05 versus sham group, P < 0.05 versus ischemia–reperfusion + melatonin group.
Figure 1. Light microscopic view of lung tissues from ischemia–reperfusion group. Extensive histological changes with inflammatory cell infiltration and partial destruction of lung architecture (magnification of 10 × 10, H&E staining).
Figure 2. Light microscopic view of lung tissues from ischemia–reperfusion + melatonin group. More preservation of nearly normal structure (magnification of 10 × 10, H&E staining).
Figure 3. Representative photomicrograph of lung tissues in the sham group showing normal structure (magnification of 10 × 10, H&E staining).
Measurement of lung water content was used to estimate pulmonary edema. Compared with the ischemia–reperfusion group, the lung water content in the ischemia–reperfusion + melatonin and sham groups was decreased significantly (P = 0.000). No significant difference in lung wet/dry weight ratio was found between the ischemia–reperfusion + melatonin and sham groups (P > 0.05).
The NO level of lung tissue was increased in the ischemia–reperfusion group (Table 1). The treatment with melatonin recovered this increase and caused a reduction in comparison with the ischemia–reperfusion group (P = 0.000). No significant difference in NO level was found between the ischemia–reperfusion + melatonin and sham groups (P > 0.05).
The novel indicator for neutrophil function, MPO activity, in the ischemia–reperfusion group was found to be increased dramatically compared to the ischemia–reperfusion + melatonin and sham groups (P = 0.000) (Table 1). MPO activity comparison between the ischemia–reperfusion + melatonin and sham groups did not show significant results (P > 0.05).
DiscussionPeripheral arterial clamping, as a surgical procedure, is routinely used for orthopedic surgery or trauma in elective and emergency cases. Lung damage has been shown to occur following transient arterial occlusion. The ischemic damage resulted from a decrease in the blood flow to an organ. When restoring blood flow a more pronounced damage, so called reperfusion injury, occurs. In the development of ischemia–reperfusion injury, the enhanced generation of oxygen radicals has been suggested.20 Free radicals have been previously demonstrated to play a significant role in the etiology of remote lung injury in animal models.4, 21 It has been widely described that lung injury often occurs after skeletal muscle ischemia due to release of free radicals of reperfusing tissues.4
Melatonin and its metabolites have been shown to have antioxidant properties in in vitro and in vivo systems, both in cells and body fluids.22, 23, 24 Furthermore, melatonin plays an important role in activating antioxidant defences,25, 26 and protects cells from oxidative loads and apoptosis induced by mitochondrial DNA deletion.27 Both effects allow melatonin to reduce the extent of reactive oxygen species, improving the outcomes of oxidative related pathologies such as ischemia35 and prevention of aging.28, 29, 30It has been demonstrated that melatonin can prevent ischemia–reperfusion injury in cerebral artery occlusion induced stroke, in traumatic brain and spinal cord injuries, in animal models.31, 32, 33 Besides the generation of free radicals, the ischemia–reperfusion injury is associated with exaggerated inflammatory response.34 Activated polymorphonuclear leukocytes aggregate and adhere to endothelium, and impair blood flow and development of endothelial cell edema.35 Recently, melatonin reduced morphological injury due to inhibition of neutrophils infiltration into the reperfused intestine with abolished P-selectin expression and intercellular adhesion molecule-1 up-regulation on endothelial cells.32
Several methods have been used to define the role of neutrophils in reperfusion tissue injury. MPO plays a fundamental role in oxidant production by neutrophils. Neutrophils are a main potential source of oxygen-free radicals.36 A previous study clearly demonstrated that skeletal muscle ischemia–reperfusion led to remote lung inflammation, which was characterized by increased MPO activity and significant neutrophil infiltration in the lung.37 NO level is a significant determinant of the lung injury process which is caused by lower extremity ischemia–reperfusion.38
The effects of melatonin on ischemia–reperfusion injury in an ex vivo rat heart model was studied by Lagneux et al.39 The authors explained that the melatonin significantly reduced infarct size and the incidence of reperfusion arrhythmias.39 In an experimental study Erkanlı et al.40 reported that melatonin has a protective effect against skeletal muscle ischemia–reperfusion injury and can reduce the incidence of compartment syndrome after chronic or acute peripheral arterial occlusions. Other studies have shown that melatonin protects kidney,41 liver,42 testis43 and intestine44 against ischemia–reperfusion injury.
In the last few years, Okutan et al.14 investigated the protective role of melatonin in lung remote organ injury. For that purpose, they induced lung injury by the occlusion of infrarenal abdominal aorta for 30 min and reperfusion for 12 h. These authors administered melatonin intraperitoneally at 20 mg/kg, 1 h prior to trial. They found that pre-treating rats with melatonin significantly decreases malondialdehyde (MDA) levels as well as MPO activity. Based on this finding, they believed that their study indicated the potential of attenuating lung injury, and require further studies to show improvement in the morphology of the injured lungs. In the current research, we tested the hypothesis that 10 mg/kg i.v. of melatonin could protect the lungs after 2 h femoral artery occlusion and 24 h reperfusion. In this study melatonin inhibited MPO activity and decreased NO level which was increased by ischemia–reperfusion. Pulmonary architecture, neutrophilic infiltration, intra-alveolar hemorrhage and edema were much reduced in the treated group. However, there are further studies to be conducted to test the effects of various doses of melatonin treatment against ischemia–reperfusion injury in lung.
Sharma et al.18 showed that lungs undergoing ischemia–reperfusion displayed increased vascular permeability and pulmonary edema. The conclusion of increased vascular permeability is an assumption based on the lung wet\dry weight ratio. Significant lung edema occurred after ischemia–reperfusion in rat which was attenuated by melatonin treatment.
Data acquired demonstrates that melatonin can significantly decrease the severity of pulmonary edema, decrease vascular permeability, decrease leukocytes infiltration and inhibits cellular apoptosis, after skeletal muscle ischemia–reperfusion. Our findings support the potential use of melatonin as a therapeutic agent in the prevention of acute lung injury associated with skeletal muscle ischemia–reperfusion.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
Received 10 September 2013
Accepted 8 January 2014
Corresponding author. dr_ashrafzadeh@yahoo.com