Dysautonomia has been independently associated with training and exercise-induced bronchoconstriction. In addition, neurogenic airway inflammation was recently associated with swimmers-asthma. We aimed to assess the relation between autonomic nervous system and airway responsiveness of asthmatic elite swimmers.
MethodsTwenty-seven elite swimmers, 11 of whom had asthma, were enrolled in this exploratory cross-sectional study. All performed spirometry with bronchodilation, skin prick tests and methacholine challenge according to the guidelines. Pupillometry was performed using PLR-200™ Pupillometer. One pupil light response curve for each eye was recorded and the mean values of pupil's maximal and minimal diameters, percentage of constriction, average and maximum constriction velocities (parasympathetic parameters), dilation velocity, and total time to recover 75% of the initial size (sympathetic parameters) were used for analysis. Asthma was defined using IOC-MC criteria; subjects were divided into airway hyperesponsiveness (AHR) severity according to methacholine PD20 in: no AHR, borderline, mild, moderate and severe AHR. Differences for pupillary parameters between groups and after categorization by AHR severity were assessed using SPSS 20.0 (p ≤ 0.05). In individuals with clinically relevant AHR, correlation between PD20 and pupillary parameters was investigated with Spearman's correlation test.
ResultsNo statistically significant differences were observed between asthmatic and non-asthmatic swimmers regarding parasympathetic parameters. When stratified by AHR, maximal and minimal diameters and percentage of constriction were significantly lower among those with severe AHR. Among swimmers with clinically relevant AHR (n = 18), PD20 correlated with parasympathetic activity: maximal (r = 0.67, p = 0.002) and minimal diameters (r = 0.75, p < 0.001), percentage of constriction (r = −0.59, p = 0.011) and latency (r = 0.490, p = 0.039).
ConclusionsNo significant differences were observed between asthmatic and non-asthmatic swimmers regarding parasympathetic parameters, but among those with relevant AHR an association was found. Although limited by the sample size, these findings support the relation between dysautonomia and AHR in asthmatic swimmers.
An increased risk for asthma has been recognized in elite athletes who take part in endurance sports, such as swimming.1 Classical postulated mechanisms behind exercise-induced asthma (EIA) include the osmotic, or airway-drying, hypothesis.2 As water is evaporated from the airway surface liquid, it becomes hyperosmolar and provides an osmotic stimulus for water to move from any cell nearby, resulting in cell shrinkage and release of inflammatory mediators that cause airway smooth muscle contraction. In fact, the airways of athletes present increased inflammatory cells and levels of histamine, cysteinyl leukotrienes and chemokines; however these inflammatory changes are not consistently related to lung function or disease exacerbations and it has been thought that they represent physical injury secondary to rigorous hyperpnoea that will heal with rest.3, 4 Alternative hypotheses to explain EIA have been pursued.
Besides inflammatory mediators, the autonomic system also mediates the contraction and relaxation of bronchial smooth muscle. Cholinergic-parasympathetic nerves stimulate bronchoconstriction, whereas β2-adrenergic sympathetic and/or noncholinergic parasympathetic nerves cause bronchodilation.3, 4 Intensive training can have affect autonomic regulation by promoting the predominance of vagal activity, as a compensatory response to the sympathetic stimulation associated with frequent and intense training.2 It has been hypothesized that repeated intensive training could provoke vagal hegemony, which induces not only the well-known resting bradycardia of athletes, but could also lead to a predisposition for increased bronchomotor tone and therefore susceptibility to bronchospasm.2 This autonomic nervous system imbalance is known as dysautonomia and it has been previously shown, using pupillometry, that pupillary light reflex of endurance runners reveals an increased parasympathetic activity and reduced sympathetic activity.5 But the relationship between these observations and asthma is not established.
Research into the hypothesis of dysautonomia in the pathogenesis of asthma in athletes is urgently needed because definite answers would allow for better targeted treatment of this specific asthmatic population. In the particular case of elite swimmers, in both asthmatic and healthy ones, an increase in bronchial responsiveness correlating with exercise intensity was demonstrated after 3000 m swimming in an indoor swimming pool.6 Moreover, neurogenic airway inflammation was recently associated with swimmers-asthma.7 Therefore, we aimed to assess the relationship between autonomic nervous system and airway responsiveness of elite swimmers with asthma. It was hypothesized that airway hyperesponsiveness in asthmatic swimmers is related to increased parasympathetic activity.
MethodsParticipantsSwimmers of the FCPorto main swimming team were invited to participate. Athletes of over 14 years-old, who agreed to take part in the study, were enrolled. To be included, participants had to be elite swimmers, free from any respiratory infection in the 2 weeks before testing, not having drunk coffee or smoked in the 2 h prior to testing, not having taken exercise on the testing day, not using contact lenses and not having taken their asthma medication for 48 h (except for inhaled corticosteroids, which they had been asked to stop taking for at least 2 weeks prior to the study).
Subjects who met any of the following criteria would have been excluded from the study: under any systemic medication which could affect the central nervous system; any topical eye treatment; systemic conditions with known ocular involvement; orbit structure damage or surrounding soft tissue with open lesion or edema on the day of testing; a past history of ocular abnormalities or trauma.
None of the subjects was excluded based on the above mentioned criteria.
Study designThis is an exploratory cross-sectional study, developed in two visits. The first visit was in the morning (from 8 to 11 am) because of the circadian rhythm of the size of the pupil.8 Medical history and potential medication were reviewed to determine eligibility. The eligible ones answered a structured questionnaire, and performed pupillometry, spirometry and skin prick testing. Subsequently, reversibility to salbutamol was evaluated. The second visit took place on a different day and a bronchial challenge with methacholine was performed to assess airway hyperesponsiveness (AHR). Asthma diagnosis was based on the typical clinical features in conjunction with objective documentation of airway dysfunction, either presenting reversibility or AHR, according to the criteria set by the International Olympic Committee to document asthma in athletes.4, 9
The study was conducted according to the Declaration of Helsinki and approved by the Ethical Commission. All participants or their legal guardians/parents (in the case of participants under 18 years-old) signed an informed consent form.
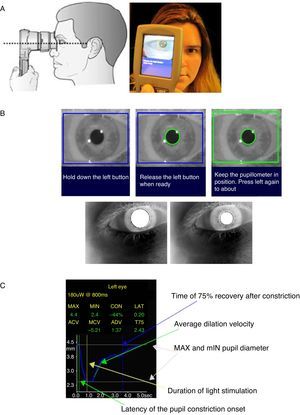
ProceduresPortable infrared PLR-200™ Pupillometer (NeurOptics Inc, CA, USA) was used for pupillary measurements. Subjects spent at least 15 min in a semi-dark and quiet room to allow their eyes to adjust to the low lighting levels, after which they were instructed to focus with the eye that was not being tested on a small target object standing at least 3 m away, keeping their head straight and eyes wide open during targeting and measurement (Figure 1A). If they blinked, the measurement was repeated. One stimulus of light-emitting diodes briefly illuminated the eye with 180 nm peak wave light (Figure 1B). At the end of the measurement cycle, a graph of the pupil diameters as a function of time appeared on the screen (Figure 1C). One pupil curve to each eye, starting with the left, was recorded for each subject, and the mean values of both eyes were used for statistical analysis. The following parameters were collected: the diameter of the pupil before (initial) and at constriction peak (minimal), in millimeters; the percentage of the constriction; the time of the onset of the constriction (latency), in seconds; the average and the maximum constriction velocities (ACV and MCV respectively), and the dilation velocity (ADV), all given in mm/s; and the total time taken by the pupil to recover 75% of the initial resting pupil size after it reached the peak of constriction (T75), in seconds. Pupil diameters, latency, ACV, MCV, and the constriction amplitude are related to parasympathetic activity, while ADV and T75 are measures of sympathetic activity.
Figure 1. Procedure for measurement of pupillary light reflexes and pupil sizes and pupillometer displaying results. (A) The sequence of figures represent the adequate position to perform the scan: at the right angle to the patient's axis of vision, in a good alignment, closely adapted to the face and the pupil in the center of LCD screen. (B) The sequence of figures presents the pupil measurement phases: targeting phase (1), ready phase (2) and measurement phase. (C) Pupillometer display of one measurement results.
Subjects underwent spirometry, which was carried out according to the American Thoracic Society criteria (ATS).10 Results of spirometry are reported as forced expiratory volume in the first second (FEV1), forced vital capacity (FVC) and forced expiratory flow in the middle portion of FVC (FEF25–75); all are presented as both absolute and predicted values, according to published reference algorithms.11 Airflow obstruction was defined as a FEV1/FVC ratio lower than 0.70.12 Lung function measurements were repeated 15 min after salbutamol inhalation (400 μg) in aerochamber to assess reversibility, which was defined as an increase on FEV1 ≥ 200 mL or 12% from baseline.12 Bronchial challenge with methacholine was performed as recommended by ATS guidelines and accordingly medication was withheld.13 Criteria for a positive challenge were, according to the International Olympic Committee, set to a provocative dose determining a 20% fall in FEV1 (PD20) ≤3.2 μmol in steroid naïve athletes and to a PD20 ≤0.8 μmol in athletes on inhaled steroids for at least 1 month.9
Subjects were divided by AHR severity according to PD20 in: no AHR (>7.8 μmol), borderline (3.2–7.8 μmol), mild (0.8–3.2 μmol), moderate (0.1–0.8 μmol) and severe (≤0.1 μmol) AHR. A PD20 ≤3.2 μmol was considered as clinically relevant AHR.
A structured questionnaire was applied that addressed demographic data, medications and medical conditions.
Atopy was defined as the presence of at least one positive skin prick test to common aeroallergen extracts (Leti®, Madrid, Spain); positive (histamine 10 mg/mL) and negative controls were performed.
Statistical analysisStatistical analyses were performed with SPSS version 20.0. p-Values <0.05 were considered statistically significant. Continuous results are expressed as mean (95% confidence interval, CI) or, if not normally distributed, as median (minimum and maximum); categorical data are expressed as counts (%). Differences between groups were assessed with Student's t-test or Mann–Whitney in cases of non-normally distributed data, and Chi-Square or Fisher's exact tests for categorical variables.
Subjects were then categorized by AHR severity and differences between groups for pupillary parameters were assessed with 1-way ANOVA or Kruskal–Wallis if non-normally distributed data.
In individuals with clinically relevant AHR, Spearman's correlation test was used to assess the relation between PD20 and pupillary parameters.
ResultsTwenty-seven elite swimmers were enrolled, of which 11 (41%) had asthma. Demographic, personal and clinical features are presented in Table 1. Regarding their normal medication, 2 swimmers were under inhaled corticosteroids, 4 with a combination of long-acting β2-agonists plus inhaled corticosteroids and 2 were treated with anti-leukotrienes. All refrained from taking their regular medication prior to the study.
Table 1. Characteristics of asthmatic and non-asthmatic swimmers.
| Asthmatic swimmers (n = 11) | Non-asthmatic swimmers (n = 16) | p | |
| Males, n (%) | 8 (73) | 6 (38) | 0.072 |
| Age (years) | 17 [15–19] | 18 [16–20] | 0.479 |
| BMI (kg/m2) | 21.5 [20.2–22.9] | 21.3 [20.2–22.3] | 0.725 |
| Atopy, n (%) | 5 (46) | 6 (38) | 0.679 a |
| Years of competition | 8.9 [7.0–10.8] | 9.6 [7.6–11.7] | 0.602 |
| Training hours per week | 16.3 [13.1–19.4] | 17.5 [16.1–18.9] | 0.393 |
| Previous diagnosis of asthma, n (%) | 4 (36) | 1 (6) | 0.113 a |
| Previous diagnosis of rhinitis, n (%) | 1 (9) | 3 (18) | 0.488 a |
| PD20 methacholine (μmol) | 0.8 [0.4–1.2] | 4.6 [3.3–5.8] | 0.001 |
| Lung function | |||
| FEV1/FVC | 83.5 [78.3–88.8] | 88.0 [84.6–91.4] | 0.236 |
| FVC | |||
| Liters | 5.1 [4.4–5.8] | 4.9 [4.1–5.7] | 0.612 |
| % of predicted | 114.5 [107.0–122.0] | 114.8 [108.5–121.0] | 0.957 |
| FEV1 | |||
| Liters | 4.3 [3.7–4.8] | 4.3 [3.7–4.9] | 0.863 |
| % of predicted | 111.1 [100.7–121.5] | 115.6 [109.8–121.3] | 0.379 |
| FEF25–75 | |||
| Liters | 4.2 [3.4–5.1] | 4.7 [4.0–5.4] | 0.570 |
| % of predicted | 97.3 [78.2–116.4] | 109.3 [97.3–121.4] | 0.230 |
| Airway obstruction, n (%) | 1 (9) | 0 | 0.219 a |
| Increase in FEV1 after salbutamol | |||
| % | 5.0 [2.0–8.0] | 3.4 [1.7–5.4] | 0.840 |
| Milliliters | 197.0 [87.2–306.8] | 149.4 [61.5–237.3] | 0.922 |
| Parasympathetic parameters | |||
| Maximal diameter (mm) | 5.9 [4.6–7.3] | 7.0 [6.4–7.6] | 0.180 |
| Minimum diameter (mm) | 3.9 [2.9–4.9] | 4.7 [4.0–5.4] | 0.708 |
| Percent of constriction | 35.1 [31.9–38.3] | 33.8 [29.3–38.4] | 0.295 |
| Latency (s) | 0.2 [0.2–0.2] | 0.2 [0.2–0.2] | 0.183 b |
| ACV (mm/s) | 4.0 [3.5–4.6] | 4.1 [3.6–4.5] | 0.664 |
| MCV (mm/s) | 5.7 [4.7–6.8] | 5.5 [4.9–6.0] | 0.592 |
| Sympathetic parameters | |||
| ADV (mm/s) | 0.9 [0.7–1.2] | 0.9 [0.8–1.0] | 0.440 |
| T75 (s) | 2.4 [1.3–3.5] | 3.1 [2.8–3.4] | 0.154 |
Data reported as mean [95% confidence interval] unless otherwise stated.
ACV: average constriction velocity; ADV: average dilation velocity; BMI: body mass index; FEV1: forced expiratory volume in the first second of FVC; FVC: forced vital capacity; FEF25–75: forced expiratory flow middle portion of FVC; MCV: Maximum constriction velocity; PD20: provocative dose determining a 20% fall in FEV1; T75: the total time taken by the pupil to recover 75% of the initial resting pupil size after it reached the peak of constriction.
In bold, p ≤ 0.05.
a Fisher Exact test.
b Mann–Whitney U test.
Pupillary response curves were easily recorded in all subjects (Figure 2). Pupillometry was repeated if blink artifacts occurred, but all participants were able to complete the measurements, except for T75 which was not retrieved by the device for all athletes. None reported discomfort.
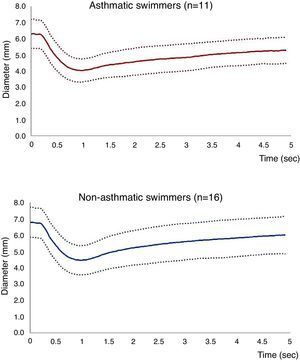
Figure 2. Results of pupillary response variables: Mean (bold line) ± SD (traced lines) values of pupillary diameters among asthmatic and non-asthmatic swimmers.
Pupillometry measurements in asthmatics compared with non-asthmatic swimmers are presented in Table 1 and Figure 2. Although lower pupil diameters and a higher percentage of constriction were observed in asthmatics, differences were not statistically significant.
When stratified by AHR severity, pupil diameters before (maximal) and at constriction peak (minimum) as well as the percentage of constriction were significantly lower among those with severe AHR (Table 2).
Table 2. Autonomic nervous system parameters across airway hyperresponsiveness (AHR) degrees of severity.
| No AHR (n = 3) | Borderline AHR (n = 6) | Mild AHR (n = 12) | Moderate AHR (n = 4) | Severe AHR (n = 2) | p | |
| Parasympathetic parameters | ||||||
| Maximal diameter (mm) | 6.4 ± 1.0 | 6.4 ± 0.9 | 6.9 ± 0.8 | 6.6 ± 0.6 | 4.9 ± 0.7 | 0.04 |
| Minimum diameter (mm) | 4.3 ± 1.1 | 3.9 ± 0.7 | 4.7 ± 0.8 | 4.2 ± 0.5 | 2.8 ± 0.7 | 0.03 |
| Percent of constriction | 34.3 ± 7.8 | 38.3 ± 2.2 | 33.3 ± 4.5 | 37.3 ± 1.9 | 42.8 ± 7.4 | 0.05 |
| Latency (s) | 2.8 (2.6–3.0) | 3.2 (2.7–3.5) | 3.1 (1.7–3.8) | 3.3 (3.3–3.3) | 1.7 (1.7–1.7) | 0.08 a |
| ACV (mm/s) | 3.6 ± 0.9 | 4.3 ± 0.4 | 4.0 ± 0.3 | 3.8 ± 0.4 | 3.5 ± 0.4 | 0.13 |
| MCV (mm/s) | 5.1 ± 0.9 | 5.8 ± 0.4 | 5.5 ± 0.6 | 5.6 ± 0.4 | 5.1 ± 1.1 | 0.52 |
| Sympathetic parameters | ||||||
| ADV (mm/s) | 0.9 ± 0.1 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.5 ± 0.3 | 0.9 ± 0.1 | 0.08 |
| T75 (s) | 2.8 ± 0.3 | 3.1 ± 0.4 | 2.8 ± 0.8 | 3.3 ± u.d. | 1.7 ± u.d. | 0.42 |
Data reported as mean ± SD, except for latency which is expressed as median (min-max).
ACV: average constriction velocity; ADV: average dilation velocity; MCV: maximum constriction velocity; T75: the total time taken by the pupil to recover 75% of the initial resting pupil size after it reached the peak of constriction; u.d.: unavailable data.
In bold, p ≤ 0.05.
a Kruskal–Wallis test.
In the 18 swimmers with clinically relevant AHR, a significant correlation was found between PD20 and maximal (r = 0.67, p = 0.002), and minimal pupil's diameters (r = 0.75, p < 0.001), percentage of constriction (r = −0.59, p = 0.011) and latency (r = 0.490, p = 0.039), but not with ACV (r = 0.243, p = 0.332), MCV (r = 0.061, p = 0.810), ADV (r = 0.075, p = 0.776), and T75 (r = 0.373, p = 0.351).
DiscussionIn our exploratory study, no significant differences were observed in parasympathetic parameters between asthmatics and non-asthmatic elite swimmers. However, for those with severe AHR a significant difference became clear, suggesting that the increased parasympathetic tonus is particularly associated with the contraction of the bronchial smooth muscle.
Previous studies supporting this hypothesis of dysautonomy associated with training match our findings. Pichon et al. demonstrated that subjects with an increased AHR had a higher vagal tone,14 which was corroborated by Park et al. findings of a relationship between AHR to methacholine and a diminished sweat secretion, tearing and salivary flow rate in healthy athletes.15 All these studies have used AHR as an outcome measure, rather than asthma status. This also seems to be the case with swimmers. Among elite competitive adolescent swimmers, for both asthmatic and healthy ones, an increase in bronchial responsiveness correlating with the exercise intensity was demonstrated after 3000 m swimming in an indoor swimming pool.6 Together with our results, this seems to point out that dysautonomia might contribute to the severity of airway reactivity in swimmers.
The lack of significant differences in parasympathetic outcomes between asthmatic and non-asthmatic swimmers is probably related to the fact that asthma a complex disease to which, besides AHR, there are many more parameters contributing. Asthma is defined as a clinical syndrome of intermittent respiratory symptoms triggered by viral infections, environmental allergens, or other stimuli, and is characterized by nonspecific airway hyperesponsiveness and inflammation.16 While bronchoconstriction is largely dependent on airway smooth muscle cells and might be related to increased parasympathetic tonus, inflammation is a multi-cellular process involving airway epithelium, eosinophils, neutrophils, lymphocytes and mast cells.17 Although in the particular case of athletes it has been proposed that inflammatory changes represent physical injury secondary to rigorous exercise,3, 4 there are several issues that are unique to this population. In athletes, two different clinical phenotypes of asthma have been suggested by Haahtela et al. The pattern of “classical asthma” characterized by early onset childhood asthma, methacholine responsiveness, atopy and signs of eosinophilic airway inflammation; and another distinct phenotype with onset of symptoms during sports career, bronchial responsiveness to eucapnic hyperventilation test and a variable association with atopic markers and eosinophilic airway inflammation.18 Our group has recently observed that athletes involved in water sports have a 3-fold increased risk of presenting the later phenotype of asthma – called “sports asthma”, not related to atopy but rather developed through their career (unpublished data). This suggests different predominant pathophysiological mechanisms of EIA in athletes and therefore a different role of the parasympathetic tonus.
In the particular case of swimming, EIA has been mainly thought to be associated with epithelial damage resulting from exposure to chloramines.19 In recent years, the observation that regular pool attendance, especially by young children, was associated with lung hyperpermeability and increased risk of developing asthma led to the “pool chlorine hypothesis”.20, 21 According to this, the increasing and largely uncontrolled exposure of young children to chlorination by-products contaminating the air of indoor swimming pools could have contributed to the childhood asthma rise in industrialized countries.20, 21 In their studies, Bernard et al. described an association between asthma prevalence and cumulated pool attendance, as well as lung hyperpermeability and total IgE levels.22 In fact, also in elite swimmers it has been shown that the endothelial cell layer, through vascular adhesion and permeability control, determines the infiltration of immune cells and leads to edema in the lungs.23 It has been hypothesized that regular attendance at chlorinated swimming pools might have a role in the development of asthma by causing an increased lung permeability, which in turn would facilitate allergen sensitization.23 We have previously demonstrated that swimmers that remain active at a 3-year follow-up significantly increase their levels of airway inflammation compared to those who quit swimming, but asthma incidence remained similar.24 Taken together all these studies suggest that the EIA explanatory model in swimmers is not only related to inflammation and allergy, but will probable include the interplay between environmental training factors including allergens and ambient conditions and the athlete's personal risk factors such as genetic and neuro-immune-endocrine determinants.2
As a major ambient factor, chloramines, and trichloramine in particular, are quite volatile and they are very easily inhaled and therefore act as potent irritants in the airways. Chemical stimulation of vagal sensory fibers by irritants reaching the lower airways can trigger tracheal and bronchial constriction, bronchospasm, mucus secretion, and neurogenic inflammation.25 This neurogenic pathway is connected to release and action of neuropeptides, such as tachykinins, including substance P, or calcitonin gene related peptide (CGRP) from primary sensory nerve terminals, by activation of transient receptor potential (TRP) channels as a response of sensory neurons to noxious stimuli.26, 27, 28 In a recent study, the role of TRP-ankyrin 1 channel on nociceptive airway sensory nerves in nonallergic AHR in relation with chloramines exposure was highlighted.7 It was demonstrated in a mouse model, that AHR can be induced by a single hypochlorite-ovalbumin instillation, independently of bronchial influx of inflammatory cells.7 These chemoreceptors are expressed on substance P-producing airway sensory nerve fibers and are involved in irritant-induced airway disease. The activation of substance P receptor leads to bronchoconstriction.28 Our results help to support the influence of the nervous system in this hypothesis of an interaction between exposure to hypochlorite and AHR independent of bronchial inflammation.
A proof of concept of this hypothesis of a higher parasympathetic activity as an etiological mechanism for bronchoconstriction in athletes would require a better responsive effect to inhaled anticholinergics, than to other drugs. It has been confirmed by practical experiments arising from Norwegian competitive endurance athletes that revealed that they respond particularly well and with a higher reversibility to inhaled ipratropium bromide than to inhaled beta2-agonists.29
Our study has important limitations. First, the reduced number of subjects included, which may prevent the study from reaching statistical significance; however, they were all elite swimmers. Secondly, the cross-sectional nature of our data. Though, it must be highlighted as a strength of our study, that a highly controlled evaluation was performed for all variables known to possibly interfere with the pupillometry.8 Also, this is the first study to address the question of dysautonomia assessed by pupillometry related with swimmers asthma and AHR. Previous studies using this method to evaluate autonomic nervous system have not addressed the relationship with bronchial outcomes. An evaluation of Portuguese Olympic athletes showed that they have higher parasympathetic control, with concomitant reduction of the sympathetic tone.30 Capão-Filipe et al. reported an increased parasympathetic activity and a reduced sympathetic activity of endurance runners when compared to other sports.5 In our study, swimmers with clinically relevant AHR, showed a significant correlation between PD20 and 4 of the parasympathetic parameters, reflecting the reliability of pupillometry, since methacholine acts as a non-selective muscarinic receptor agonist in the parasympathetic nervous system. The recent development of a portable, user-friendly, and reliable infrared pupillometer, which allows for accurate, easy, and reproducible quantitative pupillary measurements, has revived the initial enthusiasm about the clinical usage of pupillometry.7 It has been employed to evaluate a large number of conditions as it allows quantitative pupillary measurement of several parameters,7 and now has revealed itself as a reliable tool to assess parasympathetic activity in elite swimmers.
To conclude, no significant differences were observed between asthmatic and non-asthmatic swimmers regarding parasympathetic parameters, but among those with clinically relevant airway hyperresponsiveness an association was seen. Although limited by the sample size, these findings provide further support for the role of autonomic nervous system in airway responsiveness development in elite swimmers and might be a new target for future therapeutic options in this particular population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Acknowledgments
To Q-Pharma for providing methacholine for bronchial provocation challenges. To Dr. Carla Martins for her inestimable value and availability for performing bronchial provocation challenges with methacholine. To Dr. Miguel Capão-Filipe and Professor Kai-Håkon Carlsen for critical discussions of the protocol.
Received 6 March 2014
Accepted 14 May 2014
Corresponding author. marianafercouto@gmail.com