Home mechanical ventilation (HMV) represents a treatment option for patients with chronic respiratory failure and has changed prognosis and survival of many disorders in children. The aim of this study was to characterize a group of children on long-term mechanical ventilation (LTMV) for a period longer than 10 years.
MethodsA retrospective analysis was carried out including patients on LTMV for more than 10 years (LTMV-10) in a tertiary pediatric hospital. Statistical analysis: PASW Statistics 18®.
ResultsThirty-one children (61% female) belong to the LTMV-10 group. Median age at the beginning of ventilatory support was 3 years (birth to 13 years). Main indications for assisted ventilation were neuromuscular disease (n = 12, 39%), metabolic disease (n = 7, 23%) and central hypoventilation (n = 6, 19%). Volume ventilation was used in 2 children, and positive pressure ventilation in the others, mainly bilevel positive airway pressure (n = 25, 81%). Invasive ventilation via tracheostomy was used since the beginning in four cases, and subsequently in two other children. The mean time of ventilatory support was 146 months and the maximum was 219 months. Respiratory morbidity was the most frequent cause of hospitalization and the annual rate of such episodes was 0.17 per child. Global mortality rate was 19%.
ConclusionsHMV programs provide necessary and safe assistance for children with severe chronic respiratory failure. As shown in our series, it is possible to be kept on this respiratory support modality for long periods with good compliance and a small number of hospitalizations.
In the last two decades there has been an increase of children requiring long-term mechanical ventilation (LTMV) and, in many countries, programs of home mechanical ventilation (HMV) have been developed.1, 2, 3, 4, 5, 6 Several factors have contributed to this: advances in neonatal and pediatric care with improved survival of children with acute respiratory insufficiency, technological advances and development of suitable home equipment, and increased use of polysomnography (PSG) to diagnose sleep disordered breathing.5, 7 Furthermore, pressure to reduce hospital stay duration and awareness that this environment is inappropriate for children, has resulted in increased number of patients being discharged on ventilatory support.8
In this context, HMV, mainly non-invasive ventilation (NIV), provides domiciliary health care for patients with chronic respiratory failure. HMV combines significant change in family life, increased survival rate, quality of life improvement and decrease in hospital costs.3, 7, 9, 10, 11
In 1993, a HMV program for patients with chronic respiratory failure began in our hospital, with a multidisciplinary team formed by pediatric pulmonology physicians and nurses, psychologists and social workers. In the last 20 years, more than 300 children have been supported with HMV.
The aim of this study was to characterize the group of children and adolescents on HMV for a period longer than 10 years followed in our hospital and analyze compliance and impact of HMV. Patients on LTMV transferred to other institutions before a 10 years of follow-up, were excluded, although some may have kept ventilatory support.
Materials and methodsAn observational study was performed through data collection and retrospective review of medical records of pediatric patients on HMV for longer than 10 years (LTMV-10) followed in our hospital. Starting point for follow-up was day one of ventilatory support and end point was the moment of data collection conclusion (31st August 2013), patient's transferal or death.
Data collected included patient's age, gender, diagnosis, age at beginning of ventilatory support, indications for its use, ventilatory technique and mode of ventilation provided, previous overnight oximetry or PSG, length of time on HMV, patient's compliance and outcome.
Data were collected and analyzed through PASW Statistics 18®.
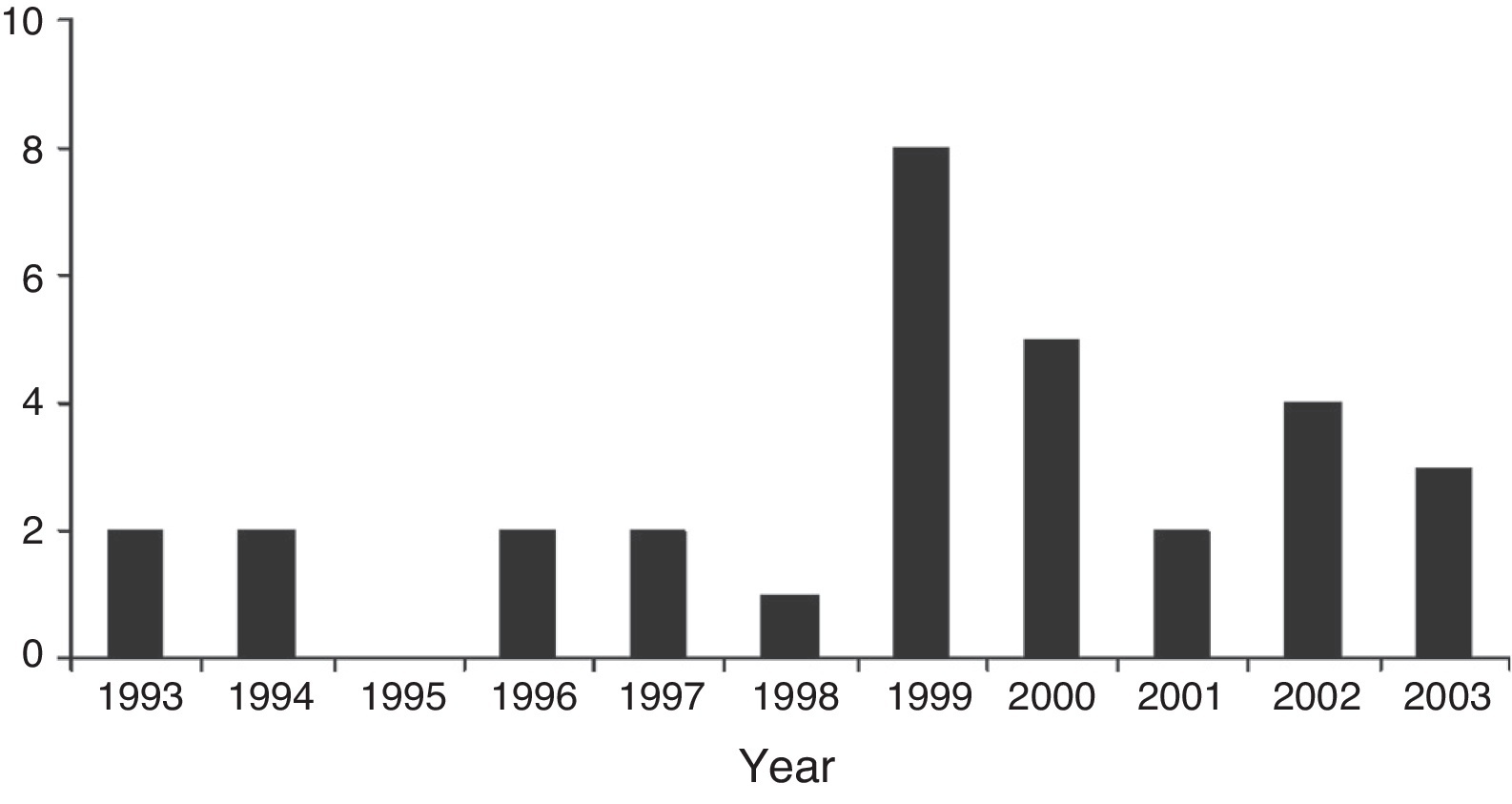
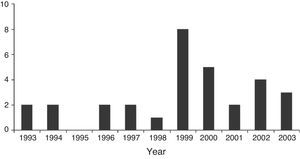
ResultsSince 1993, 31 children were under HMV for a period longer than 10 years and were followed in our hospital. Nine patients (29%) started HMV between 1993 and 1998 and 22 (71%) between 1999 and 2003 (Figure 1). The patients were categorized according to diagnosis: neuromuscular disorders (NMD) – 12 (39%), metabolic diseases – 7 (23%), central hypoventilation – 6 (19%), genetic syndromes – 2 (6%) and other neurological disorders – 4 (13%) (Table 1). Nineteen patients (61%) were female. Gender was equally distributed among all diagnostic groups.
Figure 1. Number of LTMV-10 children initiated on respiratory support per year.
Table 1. Diagnosis and age at starting of ventilatory support.
| Diagnosis | Total | Age median (min–max) |
| Neuromuscular disease | 12 | |
| CMD | 5 | 6 yr (1–8 yr) |
| SMA type II | 4 | 1.5 yr (11 mo to 9 yr) |
| SMA type III | 1 | 2 yr |
| DMD | 1 | 10 yr |
| Congenital myopathy | 1 | 6 yr |
| Metabolic disease | 7 | |
| MPS | 5 | 11 yr (3–12 yr) |
| GSD type II | 2 | 12 yr (11–13 yr) |
| Central hypoventilation | 6 | |
| Congenital (CCHS) | 5 | 0 mo |
| Acquired | 1 | 2 yr |
| Genetic syndrome | ||
| Prader-Willi syndrome | 2 | 3.5 yr (3–4 yr) |
| Other neurological disorders | 4 | |
| Hypoxic ischemic encephalopathy + BPD | 1 | 0 mo |
| Cervical spinal cord trauma | 1 | 7 yr |
| Corpus callosum agenesis | 1 | 3 yr |
| Unknown neurodegenerative disease | 1 | 8 yr |
Legend: CCHS – congenital central hypoventilation syndrome; CMD – congenital muscular dystrophy; SMA – spinal muscular atrophy; DMD – Duchenne muscular dystrophy; MPS – mucopolysaccharidosis; GSD – glycogen storage disease; BPD – bronchopulmonar dysplasia; M – months; Y – years.
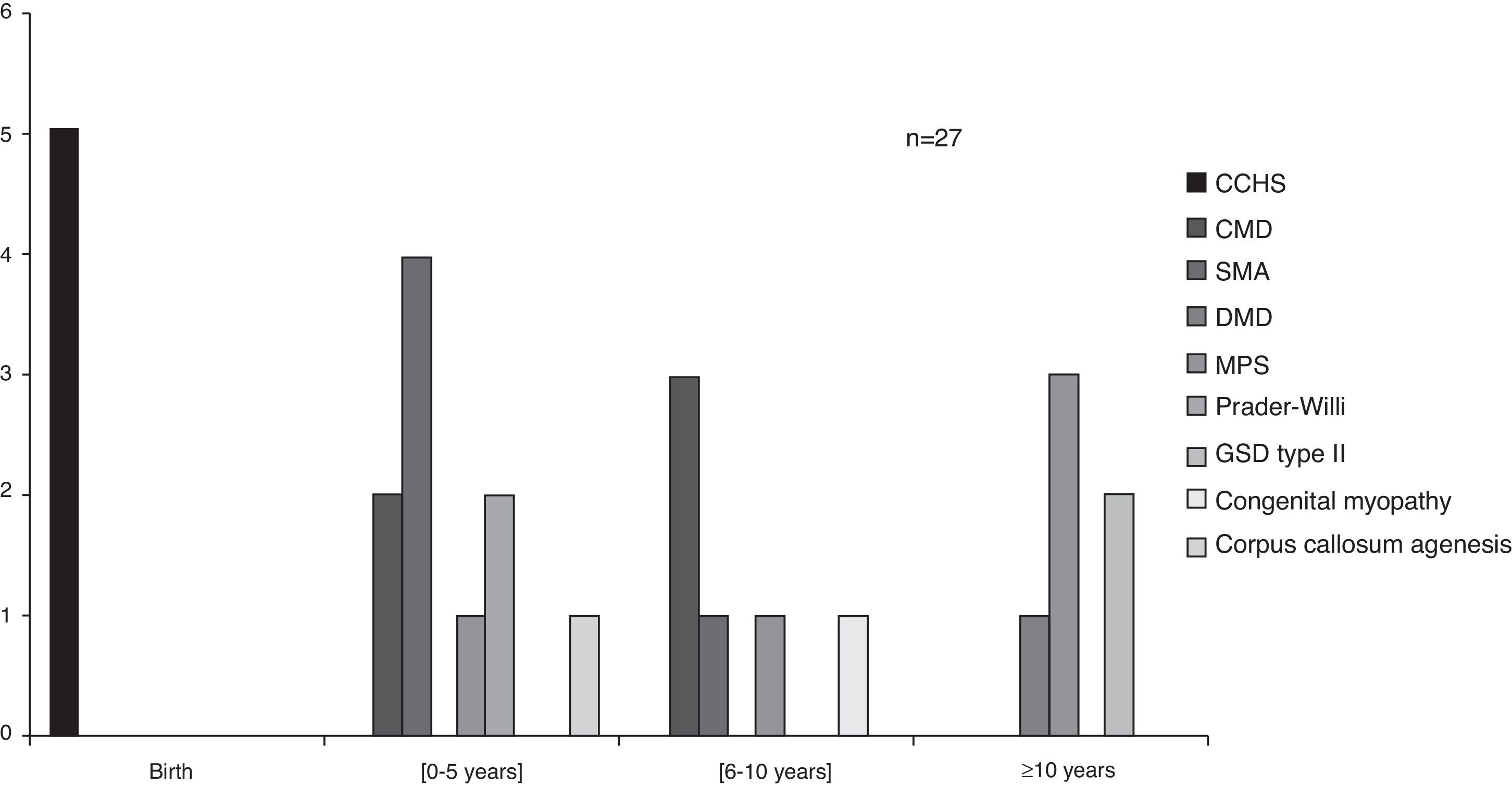
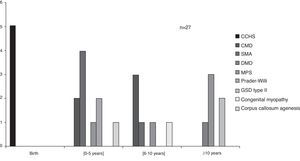
The average age of beginning ventilatory support was three years, ranging from birth to 13 years depending on the diagnosis (Figure 2). Congenital central hypoventilation syndrome (CCHS) children were subsequently on NIV, starting between 11 days and 5 months of age, with the exception of one case that stayed on invasive ventilation (tracheostomy) since birth.
Figure 2. Age at ventilatory support starting, according to congenital diagnosis. Legend: CCHS – congenital central hypoventilation syndrome; CMD – congenital muscular dystrophy; SMA – spinal muscular atrophy; DMD – Duchenne muscular dystrophy; MPS – mucopolysaccharidosis; GSD – glycogen storage disease.
Ventilatory support was started electively in 17 (55%) children (Table 2). In 12 patients, this decision was supported by a full PSG or nocturnal oximetry: PSG showed hypoventilation in 3 congenital muscular dystrophy (CMD), 1 congenital myopathy and 1 mucopolysaccharidosis (MPS) and obstructive sleep apnea (OSA) in 2 MPS, 1 Duchenne muscular dystrophy (DMD) and 1 corpus callosum agenesis; recurrent desaturations on oximetry in 1 CMD, 1 Prader-Willi syndrome (PWS) and 1 glycogen storage disease (GSD) type II. In three children with spinal muscular atrophy (SMA) type II, ventilatory support was started in the first two years of life on a prophylactic basis. Two other patients (CMD and SMA type II) started ventilatory support due to clinical symptoms of hypoventilation.
Table 2. Onset of ventilation – interface and mode of ventilation.
| Diagnosis | Total | Starting | Interface | Mode of ventilation | ||||
| Elective | Non-elective | Mask | Tracheostomy | BPAP | CPAP | Volume | ||
| Neuromuscular disease | ||||||||
| CMD | 5 | 5 | – | 5 | – | 5 | – | – |
| SMA type II | 4 | 4 | – | 4 | – | 4 | – | – |
| SMA type III | 1 | – | 1 | 1 | – | 1 | – | – |
| DMD | 1 | 1 | – | 1 | – | 0 | 1 | – |
| Congenital myopathy | 1 | 1 | – | 1 | – | 1 | – | – |
| Metabolic disease | ||||||||
| MPS | 5 | 3 | 2 | 5 | – | 2 | 2 | 1 |
| GSD type II | 2 | 1 | 1 | 2 | – | 2 | – | – |
| Central hypoventilation | ||||||||
| CCHS | 5 | – | 5 | 4 | 1 | 4 | – | 1 |
| Acquired | 1 | – | 1 | – | 1 | 1 | – | – |
| Genetic syndrome | ||||||||
| Prader-Willi syndrome | 2 | 1 | 1 | 2 | – | 1 | 1 | – |
| Other neurological disorders | ||||||||
| Hypoxic ischemic encephalopathy + BPD | 1 | – | 1 | – | 1 | 1 | – | – |
| Cervical spinal cord trauma | 1 | – | 1 | 1 | – | 1 | – | – |
| Corpus callosum agenesis | 1 | 1 | – | 1 | – | 1 | – | – |
| Unknown neurodegenerative disease | 1 | – | 1 | – | 1 | 1 | – | – |
Legend: BPD – bronchopulmonary dysplasia; CCHS – congenital central hypoventilation syndrome; CMD – congenital muscular dystrophy; SMA – spinal muscular atrophy; DMD – Duchenne muscular dystrophy; MPS – mucopolysaccharidosis; GSD – glycogen storage disease; BPD – bronchopulmonary dysplasia.
Fourteen patients (45%) had ventilatory support initiated in emergency situations. Six started in the first days of life (Table 1) and could not be weaned from ventilation and four (2 MPS, 1 NMD and 1 GSD type II) began during an acute respiratory illness. The remaining encloses one PWS with hypercapnic coma, one neurodegenerative disease requiring tracheostomy and two previously healthy children (sequelae of pneumococcal meningitis and cervical spinal cord trauma).
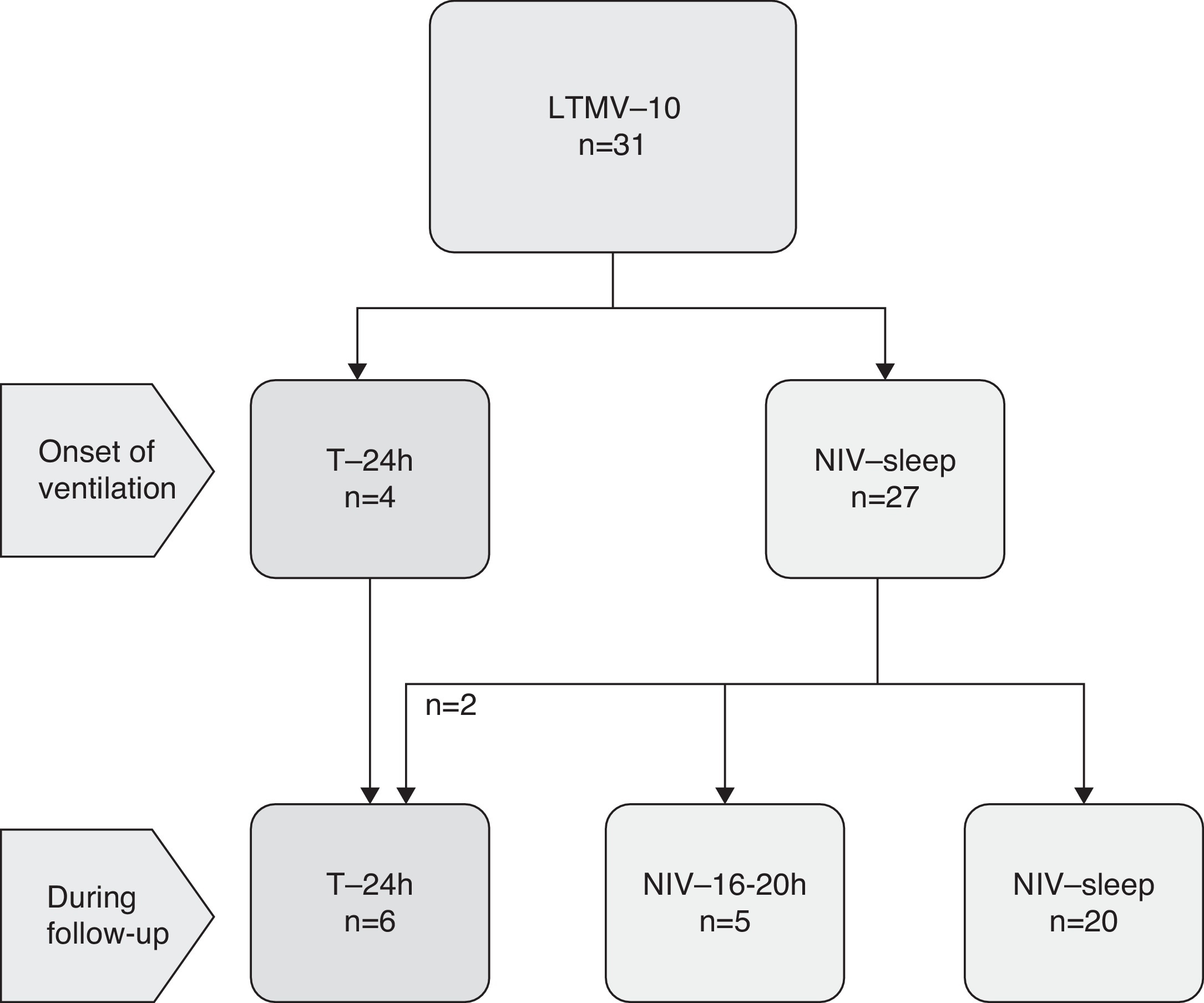
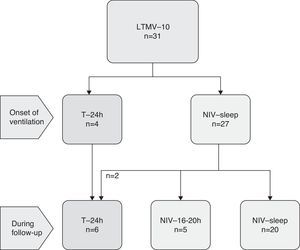
LTMV was started mainly by NIV in most children (n = 27, 87%). Invasive ventilation through tracheostomy was initiated in four children (Figure 3). During follow-up, two more patients required tracheostomy: one MPS with irreversible airway obstruction and one CMD before surgery for scoliosis.
Figure 3. Ventilatory technique on onset and follow-up. Legend: LTMV-10 – Long-term mechanical ventilation for 10 years; T – tracheostomy; NIV – non-invasive ventilation; NIV-16–20 h – NIV for 16–20 h; NIV-sleep – NIV only during sleep.
Seven children (five on NIV and the two with tracheostomy) needed to increase the daily time of ventilation to 16–20 h or full day.
Volume ventilation was used in two of the first children included and positive pressure ventilation in all the other cases, mainly bilevel positive airway pressure (BPAP) – 81% (Table 2). Continuous positive airway pressure (CPAP) was used in the four children with OSA. Three patients (1 DMD and 2 MPS) needed a subsequent change of mode of ventilation to BPAP due to association of alveolar hypoventilation.
Other support equipment was provided according to patient’ needs: oxygen saturation monitor, humidification system, suction device, mechanical in-exsufflator and self-inflating resuscitation bag.
During the last five years of follow-up, 8 (26%) patients had 5–10 respiratory infections, 12 (39%) had less than 5 and 11 (35%) had none. In the same period, 12 (39%) patients required hospitalization, mainly due to chronic respiratory failure agudization (6 NMD). The average number of hospitalizations was 0.17 per patient per year (maximum 1 per year). The first child enrolled in the study (MPS) was hospitalized 16 times during the year prior to ventilatory support initiation, 13 of which in the pediatric intensive care unit. The improvement of respiratory status after starting ventilation meant no need for admissions since then.
The mean length of LTMV was 146 months (maximum 219 months). Six children (19%) had a fatal outcome: sudden death (CCHS and MPS), acute respiratory failure (MPS), septic shock (corpus callosum agenesis), cardio-respiratory arrest (CCHS) and unknown cause (neurodegenerative disease). Twelve children made the transition to adult respiratory services. One patient was lost to follow-up.
Twelve children are currently relying on HMV and being followed in our hospital. The follow-up is performed every 3–12 months in outpatient clinics or during a programmed hospital admission. A multidisciplinary approach is performed in each evaluation, reinforcing the importance of ventilatory support, identifying and correcting problems, monitoring compliance and treating comorbidities. The overall compliance rate, based on technical ventilatory reports, is higher than 95% in each patient. Nine of the 12 children attend regular school.
DiscussionHome LTMV represents a valid and proven alternative to long-term hospitalization of children with chronic respiratory failure. Its main advantages are more humanized environment for these patients, reduction in hospital-acquired infection and decrease in health care costs.6, 12, 13
Since the beginning of the HMV program in our hospital, the number of children treated has increased substantially, a trend also seen in other series.3, 4, 5, 14, 15
One key difference between our series and others international reports are the diagnosis groups. This is related to the 10 years duration of ventilatory support. In our study there were no children with craniofacial abnormalities, airway malacia or bronchopulmonary dysplasia. These situations can improve with time due to physical growth or evolution of the disease, and so, ventilatory support is temporary.3, 6, 14, 15 In our first published series, concerning the use of domiciliary NIV during a six years’ period, 20% of 40 children included had suspended ventilation because they had improved or were cured: trachea/broncho malacia, macroglossia, diaphragmatic paresis and adenotonsillar hypertrophy associated with other situations.16 The main disorders included in the present study, such as NMD and CCHS, are much less likely to allow discontinuation of ventilatory support.11, 12, 15
We highlight how young the children of this series group were: 23% were younger than 1 year, compared with less than 10% in the Italian12 and French7 series. The median age (3 years) is also lower than in other studies (6–7 years).3, 13 This may be affected by the 4 CCHS patients who began NIV during the first year of life, which was not observed in other series.7, 12 Although a few studies have demonstrated beneficial effects from the long term use of NIV technique in CCHS course, including in children aged from 5 weeks,10, 17 its early use is not consensual. According to recent literature, these children should initiate ventilation through tracheostomy in order to achieve optimal oxygenation and neurocognitive development.18
NMD patients started HMV in different ages, as seen in other series, with median ages varying between 2 and 8 years.11, 13 In children with SMA, the prophylactic respiratory support with NIV, also seen in our series, may improve quality of life, as well as prolong life expectancy.10, 19 In SMA type I, due to its more severe prognosis, it is more difficult to balance the ethical concepts of beneficence, non-maleficence, autonomy and distributive justice.20
Six children (22%) were aged 10 years or more at onset of HMV. This group included not only children with NMD such as DMD, but also children with metabolic diseases (MPS, GSD type II) surviving through childhood. This is similar to other series,2, 5 although cystic fibrosis is also a common diagnosis in adolescents on LTMV.7 Surviving childhood, along with a cohort of young people requiring initiation of respiratory support in their teenage years, has led to a growing number of patients on HMV requiring transfer of care to adult services.15 Adult medicine physicians are now faced with medical conditions they were not previously acquainted with. In our hospital, there is a transitional program to the adult services, ensuring a smooth transfer. Regarding DMD, the only patient included was initiated on CPAP at ten years old, due to obesity associated to OSA. Some years later he was switched to BPAP. Weakness of respiratory muscles usually evolves in crescendo in adolescence, but at an early stage, mainly if obesity or adenotonsillar enlargement is associated, OSA may appear and a respiratory support may be needed.17, 21
In our series, NIV technique was used in a higher percentage than in most of other reports (26–97%).3, 4, 12, 14, 15 The major advantages of NIV, compared to invasive technique with tracheostomy, include greater patient comfort, simpler application, use and care, reduced incidence of complications7 and higher survival rates.15 The successful use of NIV has been demonstrated in children with OSA.10, 16 Furthermore, its use in children with NMD has become much more common after studies showing clear benefit in terms of outcomes and patient acceptability.2, 10, 13, 22, 23
Positive pressure ventilation was used in most cases partially because these ventilators were more available and affordable. Volume-targeted ventilation is sometimes recommended in patients with NMD,7 but for most children pressure-targeted machines work well and are simple to use.2, 13, 24, 25 The choice of the mode of ventilation depends on the medical team experience, but it is also the reflection of national ventilator company activity and other logistical or reimbursement policies.1
Similarly to other studies, respiratory support was frequently initiated during an acute exacerbation.3, 7, 12, 13, 15 These data emphasize the need to discuss LTMV during outpatient visits prior to the development of respiratory failure. In our series, the number of NMD starting ventilator support electively (86%) was higher than in other studies (approximately 50%).7, 13
Compliance to HMV was virtually 100%, a bit higher than in other series (86–97%).3, 7 As described by Ottonello et al., management of these patients requires an understanding of the specific technology involved in diagnosis and long-term care. In addition, a multidisciplinary approach is often warranted because of the complexity of the medical problems encountered in this children.12
Our results agree with published data confirming low morbidity for home care ventilator dependent children.4, 5, 11, 13 A small number of hospitalizations was seen in the majority of patients. This fact and the possibility of attending school are good indicators of improvement in quality of life.11
The overall mortality rate in our study was 19% (10–20% in other series).2, 4, 12, 14 Timely initiation of ventilatory support in children with NMD, as recommended in the recent British guidelines, may have influenced results.25
ConclusionHMV is expanding in pediatric population. As shown in our series, it is possible to keep on this respiratory support modality for long periods with good compliance, small number of hospitalizations and social reintegration. Close monitoring with a specialized team and a muldisciplinary approach, is essential for long term HMV success.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Received 16 January 2014
Accepted 28 March 2014
Corresponding author. candidacancelinha@gmail.com