Chronic Obstructive Pulmonary Disease (COPD) phenotypes have become increasingly recognized as important for grouping patients with similar presentation and/or behavior, within the heterogeneity of the disease. The primary aim of identifying phenotypes is to provide patients with the best health care possible, tailoring the therapeutic approach to each patient.
However, the identification of specific phenotypes has been hindered by several factors such as which specific attributes are relevant, which discriminant features should be used for assigning patients to specific phenotypes, and how relevant are they to the therapeutic approach, prognostic and clinical outcome. Moreover, the definition of phenotype is still not consensual. Comorbidities, risk factors, modifiable risk factors and disease severity, although not phenotypes, have impact across all COPD phenotypes.
Although there are some identified phenotypes that are fairly consensual, many others have been proposed, but currently lack validation. The on-going debate about which instruments and tests should be used in the identification and definition of phenotypes has contributed to this uncertainty.
In this paper, the authors review present knowledge regarding COPD phenotyping, discuss the role of phenotypes and comorbidities on the severity of COPD, propose new phenotypes and suggest a phenotype-based pharmacological therapeutic approach. The authors conclude that a patient-tailored treatment approach, which takes into account each patient's specific attributes and specificities, should be pursued.
Chronic Obstructive Pulmonary Disease (COPD) is a complex, multicomponent, heterogeneous disease. The classical COPD classification has been based on Forced Expiratory Volume in the first second (FEV1), but this alone is no longer accepted as a single parameter to define severity or to guide treatment.1 The updated Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations propose treatment approach based on two additional parameters, symptoms and exacerbations, which may still be insufficient to reflect the heterogeneity of COPD. There is a real need to identify specific attributes in order to group the heterogeneous COPD population into different phenotypes, and guide a patient oriented therapeutic approach. Several phenotypes have already been proposed,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 but the understanding of which attributes define which groups of patients remains a challenge.
It is recognized that future studies should focus on establishing simple algorithms based on the most discriminant features for assigning patients to specific phenotypes. Such algorithms have to be tested in validation cohorts before they can be used in clinical practice.4 Han et al. propose a schema to identify candidate phenotypes and validate them once their relevance to clinical outcomes is established.6
COPD patients frequently have several comorbidities6, 14, 15, 18, 22, 23 that should be actively sought for and duly addressed therapeutically. Some associations between comorbidities and phenotypes have been identified, and although they are relevant to the overall severity and risk, their real impact on COPD is not clearly established.
In this paper, the authors review present knowledge and accepted criteria for these crucial aspects of the disease, propose definitions for phenotyping COPD, discuss the role of phenotypes and comorbidities on the severity of COPD, and suggest a therapeutic approach for consensual phenotypes.
Phenotypes, risk factors and severityPhenotypesWith the recognition that FEV1 is not sufficient to characterize and classify COPD patients, the concept of phenotypes re-emerged, and the traditional concept of “blue bloaters” and “pink puffers”, abandoned in the past, is now being replaced by a variety of different phenotypes.24 The phenotyping process emerges as a consequence of the clinical need to group patients with similar presentation and/or behavior, within the heterogeneity of the disease, in order to provide them with the best health care possible, tailoring the therapeutic approach to each patient in terms of symptoms control, disease progression, health status and quality of life.
Han et al.6 proposed the following phenotype definition: “a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression, or death)”. It follows from this definition that patients may be classified into distinct prognostic and therapeutic subgroups. Miravitlles et al.25 simplify this definition by saying that “the term COPD phenotype is reserved for the different clinical types that have therapeutic impact and are identified in COPD patients”. Salzman proposes that an outcome can also be included,24 and some authors suggest that, depending on the context, some COPD features, e.g., dyspnea or exacerbations, could be considered both outcomes and phenotypes.26
We propose that a phenotype is an attribute or a set of attributes that can be clinically characterized, is somewhat stable over time, and define a relevant group of individuals, both in terms of therapeutic and prognostic implications.
Risk factorsThe latest GOLD guidelines contemplate the cross-combination of two distinct dimensions: a symptom dimension, assessed by the Modified Medical Research Council Dyspnea Scale (mMRC) or the COPD Assessment Test (CAT), and a risk dimension, assessed by FEV1 and frequency of exacerbations. Patients are classified as A, B, C, or D depending on the combination of these three parameters.25 However, different patients exist within the risk dimension, given that a risk assessment based on the degree of airflow obstruction is different from a risk assessment based on the occurrence of exacerbations. Moreover, exacerbations have different types, severity and presentations, and are not predictive of the same risk. Also, patients may have symptoms and risk that are independent of the respiratory disease, namely the presence of significant comorbidities.
Thus, risk and symptoms should be considered vectors or dimensions of COPD, which can be present in several phenotypes.
Some factors can be present in several phenotypes and modify the development and clinical course of COPD. In this view, comorbidities would be modifying factors since they both change over time and can exist across all phenotypes,27 and so would inflammation28 and genetic polymorphisms,11, 29 since they modify the expression of recognized phenotypes. However, no precise definition exists for modifying factors in COPD.
In COPD, the most consensual modifiable risk factor (or behavior) is smoking. Other modifiable behaviors that impact COPD are Body Mass Index (BMI)30 and physical activity.31 Environmental exposure such as exposure to air pollution can be modifiable but can hardly be considered a behavior. Furthermore, it can be argued that professional exposure is considered a modifiable risk factor, as for some patients it contributes to worsening of the disease and it can be interrupted.
SeverityHistorically, assessment of COPD severity was based solely on FEV1. Currently, and although spirometry is required to establish a diagnosis of COPD, it is considered insufficient to describe COPD severity.1, 15, 32 A comprehensive assessment of COPD including symptom assessment using validated questionnaires such as mMRC, the Clinical COPD Questionnaire (CCQ)33 and CAT,34 degree of airflow limitation, risk of exacerbations, existence of comorbidities,1 overall impact of COPD in a patient's Quality of Life (QoL), and exercise tolerance35 and levels of physical activity, is now recommended. The latter is particularly important because the amount of physical activity a patient takes and their functional status predict exacerbations, hospitalizations, and mortality.31 Indeed, the UPLIFT study has shown that although FEV1 did not differ between treatment groups (long-acting muscarinic antagonist [LAMA] vs placebo), health status, time to first exacerbation and time to exacerbation resulting in hospital admission were better in the LAMA group,36 strongly suggesting that FEV1 per se is not sufficient to determine disease severity. The TORCH study showed that FEV1 declined faster in current smokers, patients with a lower body mass index, patients with moderate disease, and patients who exacerbated more frequently,37 also suggesting that comorbidities might be major determinants of disease severity.18
COPD severity is different from COPD activity. Severity has been proposed as a concept that should be related to loss of organ function that eventually impacts on functional impairment and prognosis, whereas COPD activity relates to the activation level of the cellular mechanisms underlying disease progression.26
We propose that disease severity should be defined by mortality risk, daily impact of the disease and loss of organ function.
Identifying phenotypes in COPDDetailed questionnaire data and pulmonary function tests have been proposed to differentiate between COPD phenotypes.12 Multidimensional indexes built to stratify risk/severity are not useful in identifying different phenotypes. Nevertheless, functional measurements of severity that correlate with mortality in COPD, such as FEV1, the ratio of inspiratory capacity to total lung capacity (IC/TLC), the diffusing capacity of the lung for carbon monoxide (DLCO), 6-min walking distance, and maximum O2 consumption or maximum watts on exercise testing, may help in identifying phenotypes. Although it is not diagnostic, bronchodilator responsiveness can be useful in the distinction between asthma and COPD, and in the definition of the mixed asthma-COPD (ACOS) phenotype. However, pulmonary function tests do not identify subsets that respond to particular therapies.24
Imaging techniques, such as Computed Tomography (CT),6, 12, 38, 39, 40, 41, 42 high-resolution computed tomography (HRCT)16, 43 and magnetic resonance imaging (MRI)39 have been suggested to be of clinical use in discriminating between some COPD phenotypes, and may be novel tools that will allow for a more accurate diagnosis and help guide clinical management.39, 40 The usefulness of these techniques is still debatable, since it is recognized that there are factors not easily assessed by current techniques.41
We propose that a combination of questionnaires, objective parameters such as pulmonary function tests, including IC/TLC, 6-min walking distance, exercise testing and thoracic CT, should be able to discriminate between phenotypes.
Most clinically relevant phenotypesThe most consensual or most clinically relevant phenotypes are the non-exacerbator phenotype,14 the ACOS phenotype,8, 12, 14, 15, 17 the exacerbator with emphysema phenotype,8, 12, 14, 16 the exacerbator with chronic bronchitis,8, 12, 14, 16, 18 and the frequent exacerbator.2, 6, 7, 23 However, even these most consensual phenotypes may not be easy to manage, since considerable overlap has been described between COPD phenotypes with chronic bronchitis, emphysema or asthma, which has therapeutic consequences.12 A COPD-bronchiectasis clinical phenotype20 has also been suggested. The Spanish guidelines recognize the need to identify bronchiectasis and chronic bronchial infection in patients with the exacerbator phenotype with chronic bronchitis, but do not support them as clinical phenotypes with their own clinical relevance for the time being.14
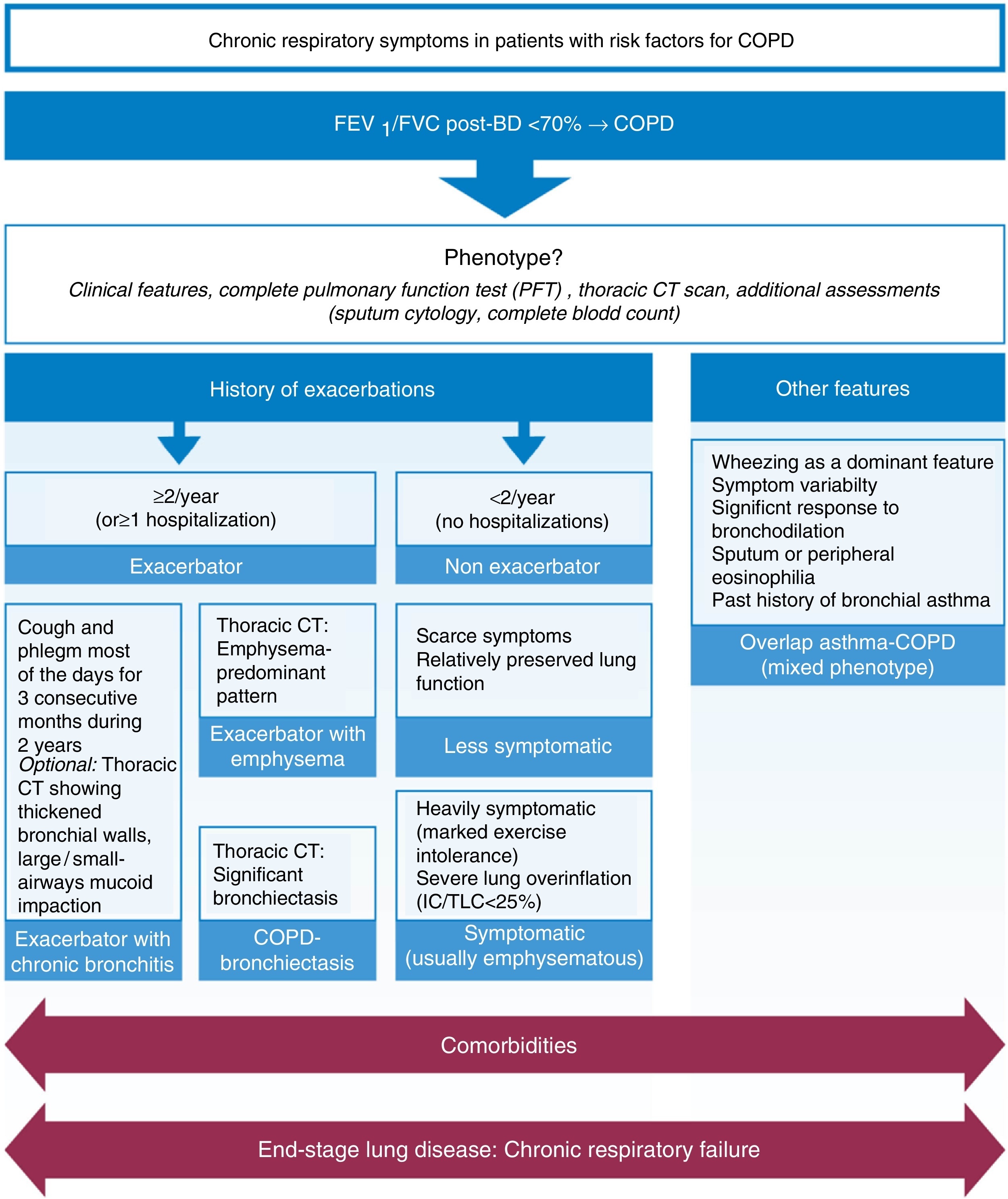
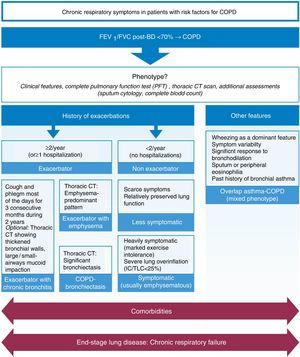
We agree with the less symptomatic non-exacerbator, the exacerbator with emphysema, the exacerbator with chronic bronchitis, and ACOS phenotypes as being four of the most clinically relevant phenotypes, propose the addition of the symptomatic non-exacerbator with emphysema as a clinically relevant phenotype, and further propose that the COPD-bronchiectasis should be considered an important clinical phenotype with its own clinical relevance – Figure 1.
Figure 1. Proposed most clinically relevant phenotypes.
Other potential phenotypesSeveral other COPD phenotypes have been proposed,3, 4, 5, 10, 11, 13, 21 but it remains unclear if these are actually specific phenotypes. The proposed fast decliner phenotype19 can only be identified over time and not a priori and thus it is difficult to include as a clinical phenotype. The combined pulmonary fibrosis and emphysema syndrome9 is another potential, albeit not consensual, phenotype, in which imaging is pivotal. Since many aspects of COPD clinical phenotypes are related to inflammation,28 it has been proposed that the presence of systemic inflammation may represent a unique COPD phenotype,6 and patients with substantial systemic inflammation and relevant comorbidities might form specific phenotypes that lead to modified treatment strategies.18 However, this is not consensual.27 Therefore, systemic inflammation and comorbidities would be modifying factors and not phenotypes. Current smokers could also represent a different phenotype, but since they must be identified across all phenotypes and intensive smoking cessation strategies must be adopted in these individuals,27 smoking would be a modifiable risk factor, or behavior, and not a phenotype. COPD severity is not a phenotypic feature since it can be a consequence of a specific clinical phenotype.26 Undoubtedly, the issue of which clinical phenotypes actually exist warrants further discussion.
Finally, it must be emphasized that comorbidities, respiratory failure and end-stage disease have impact across all COPD phenotypes.
Risk factors – significant comorbiditiesThe presence of significant comorbidities is perhaps one of the most important modifying risk factors for severity in COPD. They contribute to the overall severity in individual patients,18, 44 have a major impact on quality of life,23 increase the risk of certain causes of mortality and of all-cause mortality3, 15, 23 and are major causes of hospitalization,14, 23 especially cardiovascular disease (CVD) and cancer.3, 15, 45 They should always be considered as a very important feature in all patients, regardless of the stage or grade.
Although the prevalence of different comorbidities varies with the GOLD stage,46 available evidence does not support a relationship between comorbidities and GOLD groups. Dyspnea, health status, exacerbations and comorbidities such as chronic heart failure and depression are markedly different among subjects with the same GOLD classification, underscoring the need for a multidimensional assessment of COPD patients.3 Metabolic and cardiovascular comorbidities appear relatively unrelated to the degree of airflow limitation.4
Comorbidities can be associated with any clinical phenotype27 and should be considered in a comprehensive therapeutic approach. A few recent studies have investigated associations between comorbidities and specific COPD phenotypes,47, 48, 49, 50 or identified novel phenotypes associated with comorbidities,3, 4, 5, 21 but results are still scarce to establish associations or draw definite conclusions. The existence of COPD is associated with higher risk for other diseases, such as cardiovascular disease and lung cancer, but whether this association is due to common risk factors or common disease pathways (e.g., smoking), susceptibility genes, or impaired clearance of carcinogens is not clear.18, 23 Given the fact that more than 50% of COPD patients have four or more comorbidities,51 it is currently difficult to ascertain whether COPD is a disease distinct from its comorbidities or whether these are part of the spectrum of COPD manifestations.
Comorbidities in COPD should be managed in the same way as for all other patients without COPD.1 In fact, there is growing evidence that some of the treatments used for comorbidities, such as beta blockers, may have a beneficial effect on the course of COPD. The Spanish guidelines provide an outline of the most common comorbidities of COPD, with its diagnosis and treatment.14 Finally, whether treatment of co-morbid conditions alters the natural history of COPD or whether treatment effectiveness of COPD is altered by the presence of a concomitant comorbidity awaits further study.6, 52
Cardiovascular diseaseThe most frequent comorbidities associated with COPD are those related to the cardiovascular system.1, 15, 53, 54 The Lung Health Study reported that cardiovascular causes accounted for 42% of first hospitalizations and 44% of second hospitalizations of patients with relatively mild COPD, whereas respiratory causes accounted for only 14% of hospitalizations.15 Hypertension seems to be the most prevalent cardiovascular comorbidity1, 53 across all GOLD stages.46 COPD patients are at greater risk of cardiovascular mortality,55 with atrial fibrillation/flutter, congestive heart failure and coronary artery disease having a combined prevalence of 58.9% and being strongly associated with increased risk of death.53 Moreover, heart failure prevalence is much higher among patients experiencing an exacerbation, and is the leading cause of hospitalization and death in COPD patients.54 Also, a worsening of heart failure is a challenge in the differential diagnosis of an exacerbation.1 The presence of heart failure may be a confounding factor as well, when trying to identify a patient's phenotype. With respect to recurrent interstitial lung edema, it may be responsible for wheezing and dyspnea in the setting of chronic obstructive lung disease, thus misidentifying a patient as having ACOS.
It should be noted that different cardiovascular diseases have a different clinical impact on COPD, and the GOLD 2015 guidelines propose that four separate entities within CVD should be considered: ischemic heart disease, heart failure, atrial fibrillation and hypertension.1
Although the precise mechanisms by which COPD may be a risk factor for CVD are not fully understood, evidence has suggested that airflow limitation and particularly hyperinflation affect cardiac function and gas exchange, and that inflammatory mediators in circulation may initiate or worsen comorbidities such as ischemic heart disease and heart failure.1, 15 Hyperinflation directly affects cardiac dimensions, diastolic filling, systolic ejection fraction and cardiac mass, thus being a crucial component for some of the most relevant clinical aspects of COPD, dyspnea and exercise limitation.56, 57 On the other hand, two recent reports did not find differences in low-grade systemic inflammation between five identified comorbidity clusters, one of which was cardiovascular,51 nor confirmed that a more inflammatory COPD may be a coronary heart disease risk factor.55 One possible explanation is that inflammation is indirectly associated with cardiovascular risk, influencing other factors or comorbidities that in turn increase the cardiovascular risk.
Cardiovascular comorbidities should be treated according to usual guidelines, and beta-blockers should not be withheld from these patients, although selective beta1-blockers are preferred.1 In fact, recent studies reported a reduction of the risk for exacerbations related to beta-blockers use. The use of statins to reduce exacerbations is more controversial, and recent studies have reported different results.58, 59, 60, 61
We agree that COPD patients should be actively treated for cardiovascular comorbidities, to reduce CV exacerbations and mortality and that cardioselective beta-blockers should be used if recommended for the existing cardiovascular comorbidity.
Lung cancerA growing body of evidence supports COPD as a risk factor for lung cancer.62 However, the incidence of lung cancer among different stages of COPD has shown different results, with some studies finding increased incidence with COPD severity45 and others reporting the opposite.63 A possible justification for these contradictory results could be related to the two proposed underlying mechanisms associating COPD to lung cancer: if a patient has genetic or epigenetic risk factors common to both diseases, then lung cancer could be more prevalent at less severe COPD stages, and patients in the more severe stages would not have the predisposing risk factors; if, on the other hand, chronic inflammation is the culprit, then the worse the COPD severity, the higher the probability of developing lung cancer. However, the true reason for these different results awaits further studies.
Early lung cancer diagnosis is of paramount importance, and a recent study suggests that current and former smokers with COPD may benefit from lower pack-year threshold for lung screening eligibility.64 A potentially interesting complementary test would be to monitor circulating tumor cells, which have been shown to be detected in patients with COPD without clinically detectable lung cancer.65
Lung cancer in COPD patients should be treated according to lung cancer guidelines1.
We propose that COPD patients should be screened for lung cancer regardless of their smoking history, for early diagnosis.
Nutritional anomalies, anxiety/depression and metabolic pathologiesNutritional anomalies, metabolic disturbances and psychological disorders are three comorbidity clusters identified in COPD.51 The link between COPD and these and other frequent comorbidities may be systemic inflammation due to spillover,66 but this hypothesis remains to be fully proven.52, 67 Depression and/or anxiety are common in COPD and should be actively sought for, due to their association with increased risk of exacerbations and poorer health status.1 Lower BMI is associated with a higher prevalence of acute exacerbations46 and is an independent risk factor for mortality and poor prognosis.1 Patients with COPD frequently have one or several components of the metabolic syndrome and/or type 2 diabetes, and several mechanisms have been proposed to explain the link between COPD and metabolic disturbances. However, they are still poorly understood.68 Osteoporosis is also a very frequent comorbidity in COPD, often not diagnosed especially in men, and associated with poor health status and prognosis.
Metabolic and psychological comorbidities should be treated according to the usual guidelines, with special attention to BMI in patients with severe COPD, which should not fall below 21 kg/m2.1
We agree that these comorbidities should be actively sought for, to allow early treatment. COPD patients will benefit from a multidisciplinary treatment approach, including nutritional counseling and psychologic or psychiatric therapy.
Assessing severity in COPDThere is a need for suitable instruments to assess disease severity, so that more effective therapeutic measures can be applied. Studies suggest that, although COPD patients with more severe airflow limitation suffer more respiratory symptoms, worse quality of life and greater comorbidities than those with milder impairment, lung function alone does not adequately assess the impact of the disease.35 Nevertheless, FEV1 has to be measured since spirometry is essential for the diagnosis of COPD, to evaluate the degree of airflow limitation,1 to monitor disease progression, and to guide therapy.69 A low FEV1 is correlated with an increased risk of exacerbations1 and poor prognosis.69
As for the assessment of other parameters, several tools and other tests are currently available, such as questionnaires. The most comprehensive reliable and valid disease-specific health-related quality of life or health status questionnaires1, 34 are the St. George's Respiratory Questionnaire (SGRQ)70 and the Chronic Respiratory Disease Questionnaire (CRQ).71 However, they are both lengthy and have scoring algorithms that are too complex to use in routine clinical practice.1, 34 CAT34 or CCQ33 are shorter, practical, easy to use measures that can be completed in two minutes, and are considered suitable for a comprehensive assessment of symptoms.1 Both are valid to assess health status compared to the extensive SGRQ, but patients prefer the CCQ since it reflects their status better than CAT, as it has more details on breathing problems.72 mMRC assesses only the impact of dyspnea, but it is simple to use and recommended.1 The information provided by these questionnaires may be too scarce to accurately score COPD severity, but all questionnaires have limitations and cannot extensively include all variables relevant to assess disease severity. All these scores can be used by every physician that deals with COPD patients. One report advises caution when classifying patients according to the GOLD groups,44 since the classification obtained by the mMRC was not identical to the one obtained using CAT.73
Multicomponent indexes incorporate several dimensions of COPD. The three most commonly used multicomponent indices are BODE (BMI, FEV1, dyspnea and 6-min walking distance),30 BODEx (BMI, FEV1, dyspnea and exacerbations)74 and ADO (age, dyspnea and FEV1),75 and they are all better predictors of mortality in COPD than FEV1 alone.6 The BODE index is considered to be the reference index, the best validated and of wider use. However, the need to perform the 6-minute walking test renders it impractical in primary care, and in this setting it can be replaced by the BODEx index.74 Both indices show a high degree of correlation and a similar prognostic capacity for predicting mortality.76 In patients with more severe disease, the BODE index should always be used.14, 76 BODE seems to reflect COPD severity better than other multidimensional grading systems, but not its clinical heterogeneity.77 The ADO index seems to have a better medium- and long-term predictive reliability when compared to other indices, but after adjusting for age, BODE and BODEx have a better prognostic reliability.14 Moreover, and although ADO seems adequate to predict survival in COPD patients,75 it needs validations across a wide range of disease severities.1 The BODE Index, mBODE (BODE modified in grading of walked distance), e-BODE (BODE plus exacerbations), BODEx and the ADO index are all better predictors of mortality in COPD than FEV1 alone.
Other indexes such as the COPD Prognostic index, that predicts mortality, hospitalization, and exacerbation frequency, and the SAFE and DOSE (Dyspnea, Obstruction, Smoking, Exacerbation) indexes, which also predict exacerbations, may be useful6 as well. It has been questioned whether prediction of mortality rates in patients using indexes such as BODE and ADO truly indicates patient-perceived severity and guides appropriate treatment,18 but this is debatable since other authors argue that, being measurements of disease severity, they are useful in establishing prognosis and guiding therapy.78
All the above mentioned questionnaires and multicomponent indexes can be completed during physician appointments and are therefore subject to recall bias. Another problem inherent to the use of questionnaires is the potential to generate amounts of data that are not possible to process and interpret in due time, thus rendering the effort useless. A balance is needed between the usefulness of the information, the time it takes to collect and process, and its true impact on therapeutic choices.
There are currently no severity scores adapted to the known clinical phenotypes that can be used to guide treatment. It would certainly be desirable to have scores that better predict severity in different clinical phenotypes, and it can be expected, given the heterogeneity of COPD, that multiple variables will be needed for different COPD subtypes.19We propose the combination of several instruments to assess severity in COPD:
• FEV1 is essential.
• mMRC should be used to assess the impact of dyspnea on the patient's daily life.
• BODEx can be used in a primary care setting but BODE must be used for more advanced disease, in a respiratory care setting.
• Both CAT and CCQ are better in defining symptoms, but CAT has the added advantage of assessing disease impact.
• ADO may also be used in combination with the above, but not as a stand-alone index.
Several aspects of COPD severity are currently being challenged, and the first is, perhaps, the role of exacerbations in the clinical evolution of COPD.
An exacerbation can be defined as:
• “an event that leads a care provider to prescribe antibiotics or corticosteroids (or both) or that leads to hospitalization (severe exacerbation)”;7
• “an event that often occurs, where there is a rapid and sustained worsening of symptoms beyond normal day-to-day variations”;32
• “an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day-to-day variations and leads to a change in medication”.1
Any of the above definitions pose challenges for use in clinical phenotyping and severity evaluation. How long must changes in symptoms be sustained before being characterized as an exacerbation, is it two to three days or less? Moreover, and although the GOLD definition states: “leads to a change in medication,” the criteria invoked by health-care providers to judge when to alter medication remains unclear. Are these changes in medication quantitative or qualitative or both? Importantly, patient-recorded increases in symptoms that appear to be exacerbations outnumber those that cause them to present for medical attention. In addition, events with worsening symptoms that do not lead patients to seek additional care may also impact prognosis.6 Which clinical or biochemical markers can or should be used to identify or grade severity of exacerbations? These are unfortunately unresolved questions.
Nevertheless, and regardless of the definition, it is recognized that exacerbations contribute to the overall severity in individual patients, are associated with an increased mortality,79 the risk of exacerbations increases as airflow limitation worsens, and hospitalization for a COPD exacerbation is associated with a poorer prognosis and increased risk of death.1
Another important issue is that assessment of disease severity as envisaged in GOLD does not seem adequate. The GOLD ABCD classification results in very heterogeneous populations,80 and does not reflect disease progression or mortality risk,81, 82 as some studies have shown that GOLD B patients may be at a higher mortality risk than GOLD C patients.83 GOLD anticipates that lung function can be expected to worsen over time,1 but does not specifically state that patients may switch between GOLD categories, although an analysis from the ECLIPSE study reported that patients may indeed switch between any GOLD categories.82 It does not define dyspnea as a factor for worse prognosis, includes subsets of patients defined by low lung function with patients defined by frequent exacerbations, and does not value the presence of respiratory failure, which has important prognostic and therapeutic implications.
The non inclusion in the GOLD report of respiratory failure as a criterion for disease severity1 can have impact in clinical practice. Respiratory failure has been historically considered the hallmark of end-stage COPD, and thus, is intrinsically a severity criterion. On the other hand, is it a severity criterion only in the context of end-stage disease, or should respiratory failure be a severity criterion independent of end-stage disease?
Finally, a patient with COPD is considered to be well controlled who, during follow-up, shows minimal or no symptoms, has had no acute exacerbations since the last follow-up visit, and no impairment in QoL while receiving the current treatment.76 Therefore, in the same manner as severity assessment, disease control should be multicomponent. There is a need to intervene in the symptoms and beyond the symptoms, namely using pharmacological and non-pharmacological approaches that reduce the risk, which means, control the symptoms and control the disease beyond the symptoms.
We acknowledge that GOLD is useful in terms of general recommendations and public health, but does not take into account the several phenotypes, and is not sufficient to assess mortality risk.
Phenotype-based therapeutic approachInformation regarding specific therapeutic approaches depending on the phenotype is growing. The Canadian guidelines propose treatment based on frequent or infrequent exacerbations15 and GOLD proposes treatment based on the risk and symptoms.1 The Spanish guideline proposes treatment of COPD based on four clinical phenotypes and disease severity.14 Both physiologic measures and patient-reported outcome questionnaires will help identify these patient phenotypes and allow for optimal pharmacological treatment to be implemented.31
We suggest a phenotype-based pharmacological therapeutic approach, considering the six most clinically relevant phenotypes we have proposed – Table 1. The non-exacerbator and less symptomatic phenotype may start with a bronchodilator that, over time, will preferably be a long-acting medication. In the strongly symptomatic non-exacerbator with emphysema the aim should be maximum bronchodilation. The exacerbator phenotype should be treated first with a long-acting bronchodilator or an association of bronchodilators, to control symptoms. If not controlled, a trial of inhaled corticosteroid (ICS) association is recommended. The ACOS phenotype should be treated with LABA/ICS as first option. Whenever an ICS is recommended, the risk of increased bacterial load84 should be taken into consideration. Specific ICS drug and dose should also be appropriately chosen since there is no evidence that a higher dose produces better results and the available molecules are not equivalent.18, 84 The chronic bronchitis phenotype, if not controlled with the proposed previous treatment, can be treated with a phosphodiesterase-4-inhibitor (PDE4i – currently not available in Portugal), mucoactive drugs (acetylcysteine, erdosteine) and, if considered ineffective, also with long-term oral antibiotics (e.g. azythromycin). In the COPD-bronchiectasis phenotype, a long-term oral antibiotic should be considered and inhaled antibiotics could also be useful in patients with end-stage disease.
Table 1. Proposed phenotype-based pharmacological therapeutic approach.
| Phenotype | Therapeutic approach |
| Non-exacerbator or less symptomatic | SABA or SAMA LABA or LAMA |
| Non-exacerbator, symptomatic with emphysema | LABA + LAMA LABA + LAMA + methylxanthines |
| Exacerbator with emphysema | LAMA + LABA a LAMA + LABA + ICS LAMA + LABA + ICS + methylxanthines |
| Exacerbator with chronic bronchitis | LABA + ICS LAMA + LABA + ICS and/or PDE4i LAMA + PDE4i + cysteines |
| Mixed Asthma-COPD (ACOS) | LABA + ICS LABA + ICS + LAMA LABA + ICS + LAMA + methylxanthines |
| COPD-bronchiectasis | LABA + ICS LABA + cysteines + long term macrolide |
COPD – Chronic Obstructive Pulmonary Disease; ACOS – Asthma-COPD Overlap Syndrome; SABA – short-acting beta agonist; SAMA – short-acting muscarinic antagonist; LABA – long-acting β2-agonist; LAMA – long-acting muscarinic antagonist; ICS – inhaled corticosteroid; PDE4i – phosphodiesterase-4-inhibitor.
a Alternatively in case of a naïve patient, can initiate LAMA or LABA monotherapy, with a short-term follow-up, and in case of non-control, should progress to LABA + LAMA.
All phenotypes will benefit from non-pharmacological measures such as smoking cessation, influenza and pneumococcal vaccination, minimum of 150 min/week of moderate to intense physical activity, and pulmonary rehabilitation programs.
The proposed phenotype-based pharmacological therapeutic approach should be interpreted as a general recommendation, as some treatment options are based on expert opinion. We recommend patient education, including correct inhalation technique, prompt recognition of exacerbations, and adoption of healthy lifestyles. We strongly recommend that treatment should be patient-oriented and not COPD-oriented. A patient-tailored treatment approach, which takes into account each patient's specific attributes and specificities, should be pursued.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare collaborating and receiving fees from pharmaceutical companies other than Novartis either through participation in advisory board or consultancy meetings, congress symposia, clinical trial conduct or investigator-initiated trials.
Role of funding sourceFunding for this paper was provided by Novartis Portugal. Funding was used to access all necessary scientific bibliography and cover meeting expenses. Novartis Portugal had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.
Acknowledgements
The authors wish to thank Novartis Portugal for the funding for this paper, which was used to access all necessary scientific bibliography and cover meeting expenses.
Received 17 September 2015
Accepted 2 December 2015
Corresponding author. joaocardoso@meo.pt