The six minute walk test (6MWT) is a standardized test that provides information on exercise capacity in patients with COPD. It is considered a submaximal test in opposition to incremental cardiopulmonary exercise tests (CPET) that provide valuable information on all the systems involved in exercise.
Objectives1. To compare the perceptive, physiological responses and degree of dynamic hyperinflation during two exercise tests: the 6MWT and the incremental CPET on a treadmill. 2. To evaluate how dyspnea is related to dynamic hyperinflation (DH) and other functional parameters in both tests.
Methods29 stable COPD male patients, age 68±5.8 years, mean post-bronchodilator FEV1 57±11%, were recruited. To evaluate dynamic hyperinflation, inspiratory capacity (IC) was measured at rest and upon completing each one of the tests. At the same time, perceived dyspnea and leg discomfort were rated on specific modified Borg scales.
ResultsThe mean walk distance in 6MWT was 494±88m. The Borg scale rating for shortness of breath upon completing the test was 4.7±2, whilst 2.9±2 for leg discomfort. IC changed from 2.53±0.63l before to 2.34±0.60l after completion of the test.
In the treadmill CPET, maximal oxygen consumption (V˙O2 max) was 21.8±5mL/kg/min with 6.6±2 dyspnea and 4.3±2 leg discomfort on Borg scales. IC changed from 2.17±0.53l to 1.20±0.43l.
ConclusionsDynamic hyperinflation occurs in male COPD patients during submaximal exercise such as the 6MWT. This phenomenon is more pronounced after incremental CPET on a treadmill. Despite being dyspnea the dominant limiting symptom for both tests, we observed different physiological responses.
Patients with chronic obstructive pulmonary disease (COPD) develop dynamic hyperinflation (DH) during exercise,1,2 which has been invoked as one of the main mechanisms in the development of dyspnea.3–5
Cardiopulmonary exercise tests (CPET) provide information on the exercise capacity of these patients. Among them the 6 minute walk test (6MWT) is the most standardized field test.6 Some authors believe that 6MWT can be considered a maximum exercise test. In a study conducted in patients with moderate-to-severe COPD, Troosters et al.7 reported the 6MWT as a high-intensity submaximal exercise protocol which showed an exponential oxygen uptake (V¿O2) increase up to a plateau during the last 3min and these authors hypothesized that the self-paced walking speed during the test is set to achieve “maximal” sustainable exercise. These data are quite similar to those found by Casas.8 On the other hand, in the pulmonary function laboratory setting, incremental CPET on a treadmill is regarded as a more accurate way to reflect daily life exercise.9–12 Despite being based on the same type of exercise and not differences made for exercise performance evaluation, few studies have compared the 6MWT and the incremental CPET on a treadmill in COPD patients.13,14
The purpose of this study is to compare the perceptive, physiological responses and degree of dynamic hyperinflation in 6MWT and incremental CPET on a treadmill; and to evaluate how dyspnea is related to dynamic hyperinflation (DH) and other functional parameters in both tests.
Material and methodsStudy populationMale patients with moderate to severe COPD from our outpatient clinic were selected. They had to meet diagnostic criteria for COPD according to GOLD in stages 2–4. To be included, patients had to have been clinically stable on the previous 2 months and had to be free of other conditions limiting exercise capacity. No contraindication to undertake a CPET according to the SEPAR proceedings (Spanish Society of Pathology of the Respiratory System) should be present.15 Patients had to provide written consent to be included in the study, and for each single test the usual information was provided by laboratory staff.
The study was approved by the Hospital's Research Ethics Committee.
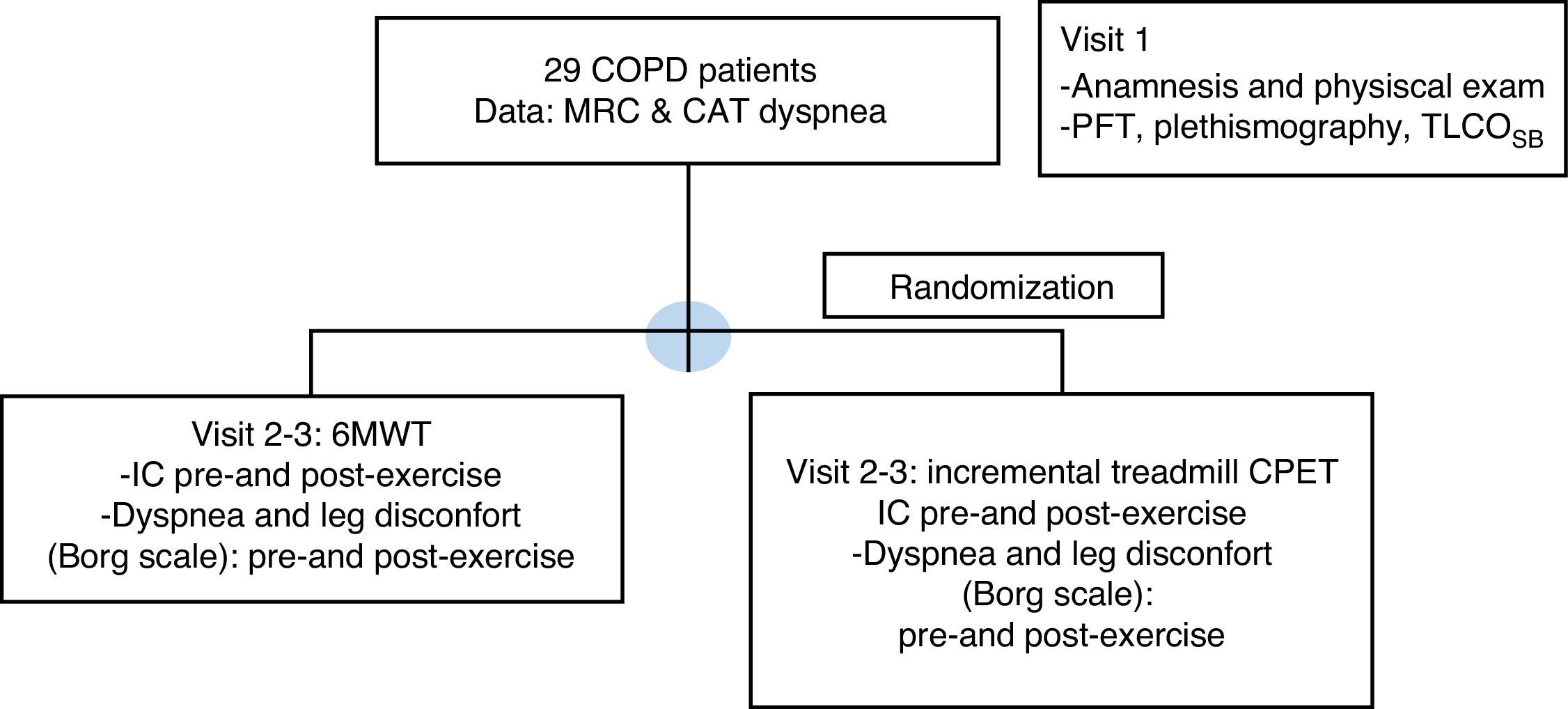
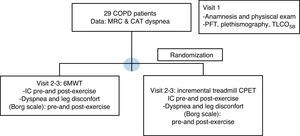
Study designPatients included were evaluated in three separate visits as is shown in Fig. 1: a run-in inclusion visit and two separate exercise test visits.
Consort flow diagram. COPD: chronic obstructive pulmonary disease; MRC: Medical Research Council; CAT: COPD Assessment Test; 6MWT: six minute walk test; BMI: body mass index; IC: inspiratory capacity; CPET: cardio-pulmonary exercise test; PFT: pulmonary function tests; TLCOSB: single breath CO diffusion test.
During the first visit, suitable patients were interviewed and included in the study if they met inclusion criteria and gave written consent to participate in the study and to perform the tests. Thorough clinical interview and physical exam were performed. Pulmonary function tests (PFT), at baseline and after bronchodilation were performed, including a plethysmography and a single-breath CO diffusion test (TLCOSB). CAT (COPD Assessment Test) questionnaire was filled in and patient's dyspnea was rated according to the mMRC (modified Medical Research Council) scale.
The second and third visits were scheduled in a period of 5–7 days to perform the incremental treadmill CPET and the 6MWT, in a random order determined by a computer based randomization method.
ProceduresPulmonary function testsAll measurements were taken according to SEPAR guidelines and European Respiratory Society (ERS) reference values were used.16,17 Forced spirometry, carbon monoxide diffusion capacity (TLCOSB) and plethysmography measurements were performed with Master Lab C Jaeger Care Fusion equipment (Würzbug, Germany®).
Symptom perceptionDyspnea was rated according to the mMRC scale. Prior to each exercise test and at the completion of the tests, patients were asked to indicate their shortness of breath and leg discomfort using a modified Borg dyspnea scale.18
Inspiratory capacity (IC)IC was determined prior to starting both tests, during rest (pre-exercise IC) and immediately after finishing the exercise (post-exercise IC). All patients had been previously instructed on how to perform the maneuver. Patients were always supervised by technicians with experience in this maneuver.19
IC was performed as described by Guenette.2 After performing four to six tidal breaths, with the patient at functional residual capacity (FRC), he was encouraged to breathe in as much air as possible, up to his total lung capacity (TLC). On the expiration preceding each maneuver, the patient was notified that a full inspiration maneuver would be required for the next breath. Approximately 1min after such a determination, and if respiratory pattern maintained constant tidal breathing, the patient was encouraged to undertake another inspiratory capacity maneuver. Three maneuvers with variability less than ±10% or ±100mL were considered reproducible. So, maximally three maneuvers were performed, accepting the maximal value obtained as IC, which was expressed in absolute values (liters).
The IC maneuver carried out before and after the treadmill CPET was performed with a pre-fitted mask and in standing position, which is an adaptation of standard specifications for the technique according to Guenette, using the Oxycon delta ergospirometer employed during the test, whilst for the 6MWT manoeuvers were performed with a non-portable spirometer (MasterLab C Jaeger, CareFusion, Würzbug, Germany®) with the patient seated. Only the first IC attempts performed immediately after completing exercise tests were accepted as post-exercise IC.
Exercise tests6MWT: it was performed according to SEPAR guidelines for cardiopulmonary exercise testing.15 Each subject performed two tests with a minimal rest period between tests of 20min. The test with the longest distance was accepted as test result.
CPET on treadmill: this test was performed using an Oxycon Delta Jaeger CareFusion ergospirometer (Würzbug Germany®). Oxygen uptake and carbon dioxide output (V˙O2 max and V˙CO2), ventilatory equivalent (Eq) and ventilation (V′E) values were obtained. SpO2 and 12-lead ECG were used for continuous monitoring. The incremental protocol based on treadmill speed and gradient changes according to Porszasz was followed.20 The procedure established that the test should progress up to limitation by symptoms, unless no other circumstance justified its interruption (alteration in the ECG, SpO2<85%).
Comparison of both exercise tests were based on parameters common to both of them: heart rate (HR), oxygen saturation (SpO2) determined with pulse oximeter, inspiratory capacity (IC) changes, dyspnea, and leg discomfort graded on Borg scales.
Statistical analysisData are shown as arithmetic mean (standard deviation). IC is expressed in absolute values, in liters. Qualitative variables were compared using the Wilcoxon test given the non-normality of the sample. Furthermore we used the bootstrap procedure21,22 to obtain the differences between means and the simulated p-values. We generated 10000 bootstrap samples (with replacement). Critical values are obtained using the standard bootstrap percentile test procedure, which retains the essentially non-parametric nature of the bootstrap approach without imposing parametric assumptions on the distribution.
Post-exercise dyspnea and 6 MWD (dependent variables) were compared with baseline clinical and lung function values (independent variables) using the Pearson correlation coefficient and we applied the bootstrap procedure to obtain the correlation coefficient and the simulated p-values. A value p<0.05 was considered statistically significant. The Statistical Package for Social Sciences (SPSS), version 20.0, software program was used for analysis.
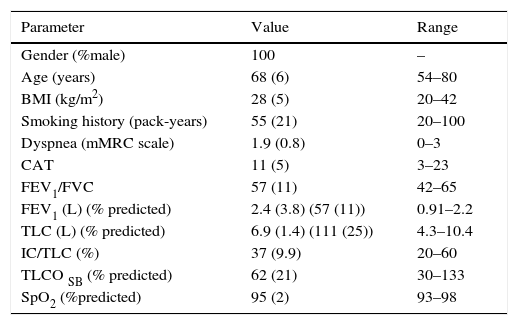
ResultsGeneral characteristicsA total of 29 COPD male patients were included; all of them male, age 68 (6) years; history of smoking of 55 (21) pack-years. None of the patients were active smokers. Patient baseline characteristics are listed in Table 1.
Characteristics of patients studied.
| Parameter | Value | Range |
|---|---|---|
| Gender (%male) | 100 | – |
| Age (years) | 68 (6) | 54–80 |
| BMI (kg/m2) | 28 (5) | 20–42 |
| Smoking history (pack-years) | 55 (21) | 20–100 |
| Dyspnea (mMRC scale) | 1.9 (0.8) | 0–3 |
| CAT | 11 (5) | 3–23 |
| FEV1/FVC | 57 (11) | 42–65 |
| FEV1 (L) (% predicted) | 2.4 (3.8) (57 (11)) | 0.91–2.2 |
| TLC (L) (% predicted) | 6.9 (1.4) (111 (25)) | 4.3–10.4 |
| IC/TLC (%) | 37 (9.9) | 20–60 |
| TLCO SB (% predicted) | 62 (21) | 30–133 |
| SpO2 (%predicted) | 95 (2) | 93–98 |
Mean (standard deviation) for each value or percentage (%) are shown.
FEV1/FVC and FEV1 values refer to post bronchodilation values.
BMI: body mass index; CAT: COPD Assessment Test; FEV1: forced expiratory volume in 1s; TLC: total lung capacity, IC: inspiratory capacity at rest, TLCOSB: single-breath diffusing capacity for carbon monoxide. SpO2: oxygen saturation determined by pulse oxymeter.
All patients were treated with a long acting anticholinergic (LAMA); 18 patients were also being treated with a combination of a long-acting beta-agonist (LABA) and inhaled corticosteroid and 11 patients with a LABA-LAMA association.
Relevant non-respiratory comorbidities were the following: 12 patients were diagnosed with atrial fibrillation, 6 had a history of ischemic heart disease. None of the patients had a disease of the nervous or musculoskeletal system.
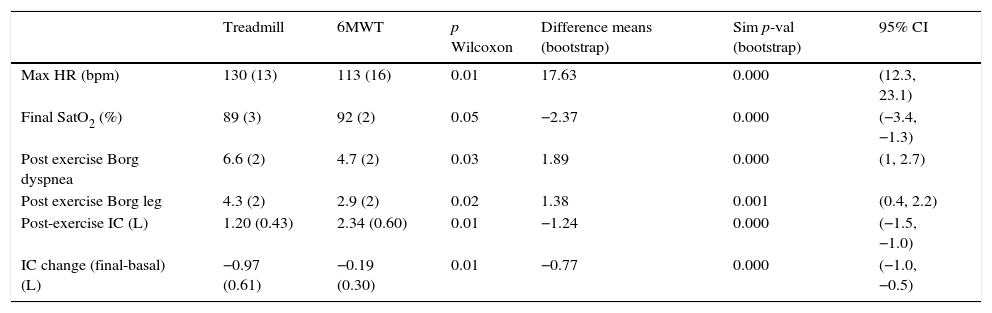
Physiological differences between 6MWT and treadmill testsAs we can read from Table 2 significant differences in functional and perception parameters can be identified between both tests. The Wilcoxon test, the difference in means with the simulated p-value using bootstrap and the confidence intervals (95% CI) are also shown in Table 2. Based on higher heart rate, lower SpO2 and post IC, as well as higher dyspnea perception, the final effort on the treadmill CPET can be considered more intense.
Physiological differences between 6MWT and treadmill.
| Treadmill | 6MWT | p Wilcoxon | Difference means (bootstrap) | Sim p-val (bootstrap) | 95% CI | |
|---|---|---|---|---|---|---|
| Max HR (bpm) | 130 (13) | 113 (16) | 0.01 | 17.63 | 0.000 | (12.3, 23.1) |
| Final SatO2 (%) | 89 (3) | 92 (2) | 0.05 | −2.37 | 0.000 | (−3.4, −1.3) |
| Post exercise Borg dyspnea | 6.6 (2) | 4.7 (2) | 0.03 | 1.89 | 0.000 | (1, 2.7) |
| Post exercise Borg leg | 4.3 (2) | 2.9 (2) | 0.02 | 1.38 | 0.001 | (0.4, 2.2) |
| Post-exercise IC (L) | 1.20 (0.43) | 2.34 (0.60) | 0.01 | −1.24 | 0.000 | (−1.5, −1.0) |
| IC change (final-basal) (L) | −0.97 (0.61) | −0.19 (0.30) | 0.01 | −0.77 | 0.000 | (−1.0, −0.5) |
The mean (standard deviation) of each value and percentage (%), when appropriate, is shown. The Wilcoxon test (p), the difference in means and the simulated p-value (sim p-val) using bootstrap are shown. 95% CI: 95% confidence intervals of variables shown. Max HR (bpm): maximum heart rate (beats per minute), SpO2: oxygen saturation, IC: inspiratory capacity, IC change: post exercise IC values−pre exercise IC values.
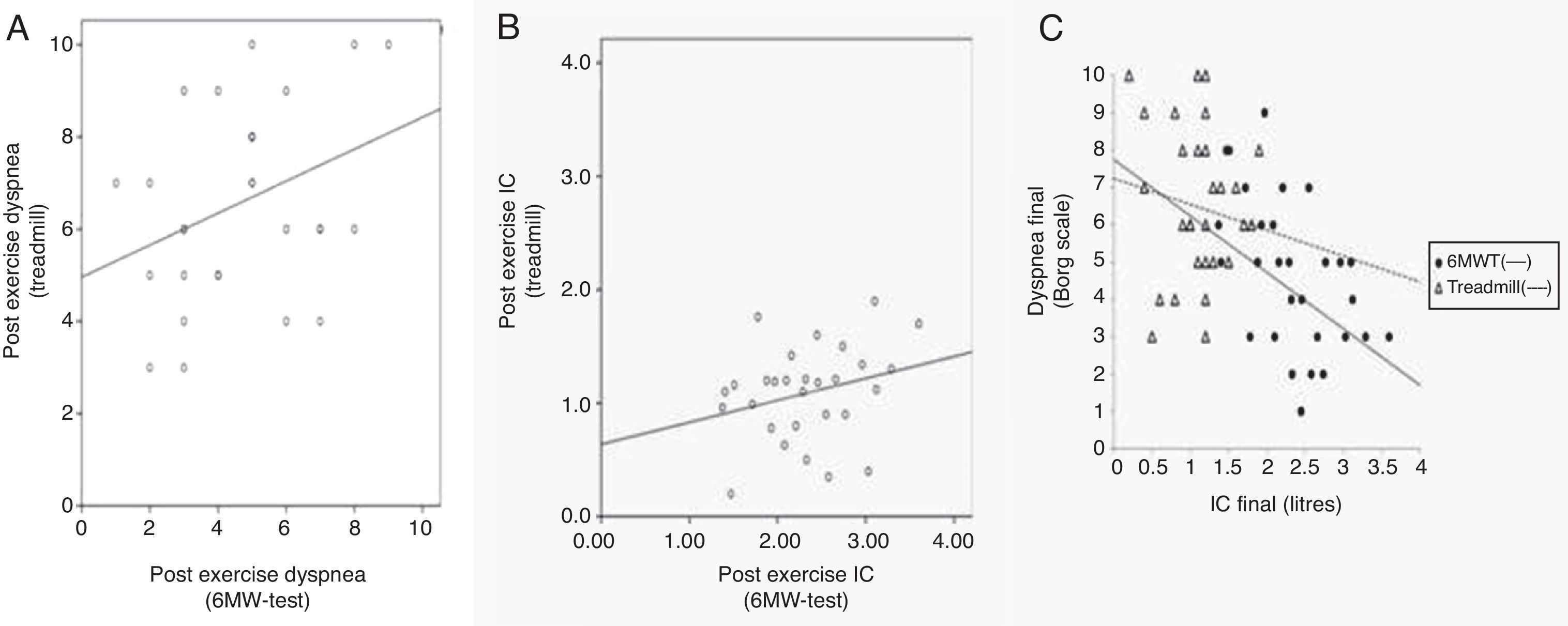
If we compare both tests regarding post-exercise dyspnea, we observe a statistically significant correlation (r=0.34, p 0.046), shown in Fig. 2A. Post-exercise IC values for both tests are represented in Fig. 2B, not showing a statistically significant correlation (r=0.27, p 0.15).
Differences in 6MWT and treadmill CPET. (A) Post-exercise dyspnea after 6MW-test (x axis) and after treadmill CPET (y axis). r: Pearson's correlation coefficient. r: 0.34; p 0.046. (B) Inspiratory capacity (IC) values upon each exercise test expressed in liters. X axis refers to 6MWT values and Y axis to IC values after the treadmill incremental CPET. r: Pearson's correlation coefficient. r: 0.27; p 0.15. (C) Dyspnea (y axis) and IC (x axis) when completing the incremental treadmill CPET (triangles) and 6MW-test (circles). IC is expressed in liters and dyspnea is as rated in Borg scale.
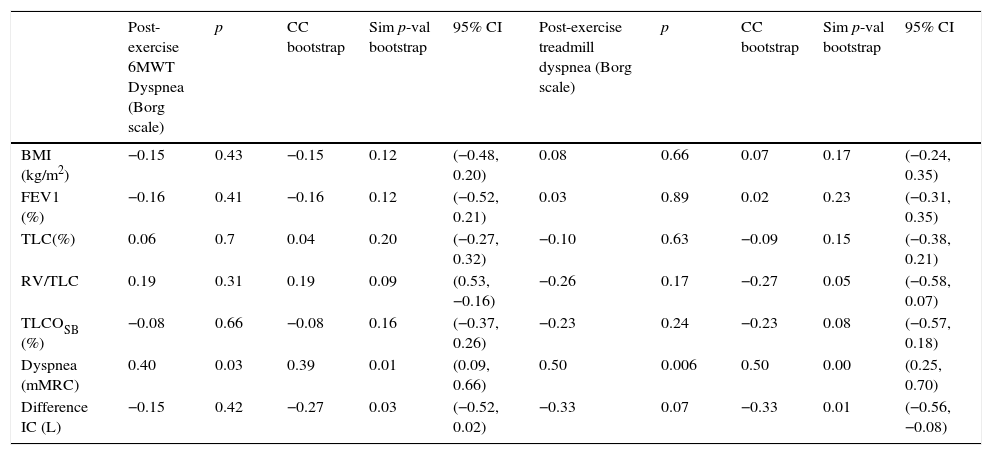
As shown in Table 3, the dyspnea perceived upon completing the 6MWT is positively correlated with baseline dyspnea according to the mMRC (r=0.39, sim p-val 0.01) and negatively correlated with the difference of IC (r=−0.27, sim p-val 0.03) and with post-exercise IC (r=−0.52, p 0.004) as shown in Fig. 2C (circles). Post-exercise dyspnea was not related with other examined parameters, included the bootstrap procedure: BMI (r=−0.15, p 0.43), percentage of predicted FEV1 (FEV1%) (r=−0.16, p 0.41), TLC (%) (r=0.06, p 0.7), RV/TLC (r=0.19, p 0.31) or TLCOSB (%) (r=−0.08, p 0.66).
Correlation of post-exercise dyspnea upon both tests with other functional parameters.
| Post-exercise 6MWT Dyspnea (Borg scale) | p | CC bootstrap | Sim p-val bootstrap | 95% CI | Post-exercise treadmill dyspnea (Borg scale) | p | CC bootstrap | Sim p-val bootstrap | 95% CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | −0.15 | 0.43 | −0.15 | 0.12 | (−0.48, 0.20) | 0.08 | 0.66 | 0.07 | 0.17 | (−0.24, 0.35) |
| FEV1 (%) | −0.16 | 0.41 | −0.16 | 0.12 | (−0.52, 0.21) | 0.03 | 0.89 | 0.02 | 0.23 | (−0.31, 0.35) |
| TLC(%) | 0.06 | 0.7 | 0.04 | 0.20 | (−0.27, 0.32) | −0.10 | 0.63 | −0.09 | 0.15 | (−0.38, 0.21) |
| RV/TLC | 0.19 | 0.31 | 0.19 | 0.09 | (0.53, −0.16) | −0.26 | 0.17 | −0.27 | 0.05 | (−0.58, 0.07) |
| TLCOSB (%) | −0.08 | 0.66 | −0.08 | 0.16 | (−0.37, 0.26) | −0.23 | 0.24 | −0.23 | 0.08 | (−0.57, 0.18) |
| Dyspnea (mMRC) | 0.40 | 0.03 | 0.39 | 0.01 | (0.09, 0.66) | 0.50 | 0.006 | 0.50 | 0.00 | (0.25, 0.70) |
| Difference IC (L) | −0.15 | 0.42 | −0.27 | 0.03 | (−0.52, 0.02) | −0.33 | 0.07 | −0.33 | 0.01 | (−0.56, −0.08) |
Values from columns 2 and 7 are Pearson's correlation coefficients. CC bootstrap is the correlation coefficient using bootstrap procedure and the simulated p-value (sim p-val) using bootstrap. 95% CI: 95% confidence intervals of variables shown.
BMI: body mass index; CAT: COPD Assessment Test; post FEV1: post-bronchodilator forced expiratory volume in 1s; post TLC: post-bronchodilator total lung capacity, RV: residual volume, TLCOSB: single-breath diffusing capacity for carbon; BMI: body mass index; CAT: COPD Assessment Test; post FEV1: post-bronchodilator forced expiratory volume in 1s; post TLC: post-bronchodilator total lung capacity, RV: residual volume, TLCOSB: single-breath diffusing capacity for carbon monoxide. Difference IC: IC at the end of exercise−IC at the beginning of the exercise.
Similar to 6MWT, for the incremental treadmill CPET and as it is shown in Table 3, mMRC dyspnea was significantly linked (r=0.5, p 0.006) with the dyspnea upon finishing this test. We observed a statistically significant relationship between dyspnea and the difference of IC with bootstrap procedure (r=−0.33, sim p-val 0.01) and with RV/TLC (r=−0.27, sim p-val 0.05). In Fig. 2C (triangles) is graphically shown the dyspnea completing the treadmill with the final IC.
No statistically significant correlation was found between post-exercise dyspnea completing the treadmill and BMI (r=0.08, p 0.66), percentage of predicted FEV1 (r=0.03, p 0.89), TLC (%) (r=−0.10, p 0.63) or TLCOSB (%) (r=−0.23, p 0.24).
DiscussionFrom the results obtained in our study we can conclude that both types of walking exercise, continuous 6 minute walking and incremental workload CPET on a treadmill, entail development of dynamic hyperinflation (DH) which can be determined by measuring IC. Nevertheless, the different degree of DH on both tests, which is higher after the incremental CPET on a treadmill, can be attributed to different exercise intensities expressed by differences in heart rate, oxygen desaturation and symptoms. These data indicate that different exercise protocols elicit different responses, so that equivalences between test results are hard to make and that, in clinical practice, some clinical changes might be easier to demonstrate with one test modality and not with another.
In COPD patients, intolerance to exercise has a multifactorial etiology.23 The factors that contribute to this reduced capacity for exercise are peripheral vascular dysfunction, abnormalities in oxygen transport, ventilatory inefficiency, development of dynamic hyperinflation and resulting limitation because of symptoms (shortness of breath or leg discomfort).4 Hyperinflation as a cause of limitation to exercise is an accepted phenomenon demonstrated by different authors.5 In COPD patients, when respiratory frequency increases during physical exercise and expiratory time shortens, there is a gradual air entrapment that leads to dynamic hyperinflation, with a progressive decrease in IC. Determination of IC at rest and during exercise therefore provides valuable information on the COPD patient's ventilatory changes during exercise.24 In our study we have verified that there is a much higher degree of DH for the incremental treadmill CPET compared to the 6MWT, with a mean loss of 970mL and 190mL in IC after performing either test, respectively. We found a significant, inverse relation between post-exercise breathlessness and IC on both tests. A priori, these results could explain appearance of dyspnea upon both tests and the more intense sensation after the treadmill CPET compared to the 6MWT (6.6/10 vs 4.3/10 respectively). Nevertheless, there is no tight link between both parameters, as can be seen in Fig. 2C, where patients with quite similar levels of IC could have a quite different dyspnea sensation, resulting in a large scatter of the points. Classically, several mechanisms have been involved in the development of dyspnea during exercise in COPD.25,26 Based on the data of our study, we can confirm that although DH seems to be an important factor in the genesis of dyspnea in our patients, it is probably not the determinant factor for this complex sensation, particularly in steady state exercise during a 6MWT. There also exists a great deal of variability regarding dyspnea perception in patients after an intense incremental treadmill exercise, not closely related to DH, as can be seen in Fig. 2C.
Pepin and co-workers demonstrated that perceptual response was consistent with physiological findings.12,25 However, there are increasingly recognized discrepancies between pulmonary function data and symptoms in COPD patients, emphasizing the value of performing exercise testing. In our study, the only parameter predictive for dyspnea at the end of the different exercise tests was baseline mMRC dyspnea rating: (r=0.4, p 0.03 for 6MW-test and r=0.5, p 0.006 for the treadmill). We did not find a relationship (just in RV/TLC on the treadmill) between static functional parameters and different perception of dyspnea upon completing both tests. This fact underlines the known disproportions between different areas of disease involvement, namely between functional impairment and perceptual component in our case.
Besides the type of exercise, there is little information on studies that have compared different exercise testing protocols. Palange and colleagues have compared walking and cycling and demonstrated a greater level of breathlessness and lower leg fatigue after an incremental shuttle walking test (ISW) compared with an incremental cycling protocol.11 Other authors such as Mathur and co-workers found that dyspnea was the major symptom limiting both types of exercise when comparing incremental walking on a treadmill with incremental cycling.14 The finding that dyspnea is the primary symptom limiting walking exercise raises the question of whether a field 6MWT may be more appropriate or more sensitive to change when assessing the effects of therapeutic interventions on exercise-induced dyspnea.27,28 Marin et al. studied the development of DH during the 6MWT.29 In their study, they measured IC during the 6MW-test. This work established an inverse relation between IC and the sensation of shortness of breath reported at the end of the test (r=−0.49, p<0.00001), similar to the results of our study.
The responses found in our study in both tests enable us to state that they are not equivalent. We observed a maximal HR achieved during the treadmill CPET (HR treadmill=130±13bpm) that was higher than in the 6MWT (HR 6MWT=113±16bpm). SpO2 at completion of the treadmill CPET (SpO2=89±3%) was lower than when completing the 6MWT (SpO2=92±2%). Based on these data, we conclude that test intensities were not comparable, so that the 6MWT can be considered a submaximal test, contrary to what authors such as Casas have argued in the literature.8 These authors have evaluated patients with moderate to severe COPD and found that during the 6MWT patients showed an increase of oxygen uptake (V˙O2 max) up to a plateau maintained during the last 3min of the test. A similar physiological response was shown at critical walking speed (CWS), which indicates that patients with moderate to severe COPD set their walking speed during the test in order to achieve the maximal sustainable V˙O2, so that this test is considered a maximal stress. In the aforementioned study 8 COPD patients (FEV1=50±13%) were included; they underwent different exercise tests with different protocols. During the 6MWT they obtained a HR=130±18bpm and dyspnea measured with the Borg scale was 5.4±1.3. In our patients no such data of maximal cardio-respiratory stress could be observed during the 6MWT, in contrast to incremental treadmill CPET.
This study has several limitations. For instance, the COPD patients we studied may not always be homogenous between studies as we can suspect from discrepancies between aforementioned references or from striking differences between individuals. We have only included male patients, more prevalent in Spanish COPD populations, therefore our results cannot be extended for all COPD patients. The small number of patients included could be another limitation but the statistical analysis we have used, the bootstrap procedure, compensate for the limitation of this small number of patients included in the study.
IC was only evaluated prior to starting the exercise and at the end of the test. It was not possible to evaluate the response throughout the tests. This can be justified by the complexity of achieving reliable manoeuvers with acceptable reproducibility. Interbedded manoeuvers raise the possibility of changing DH levels and alter the perception of symptoms during the test, which could be impaired with respect to their usual performance.30,31 The degree of DH during the 6MWT may also have been underestimated because of the time taken by the patient upon completing the test to approach the spirometer to perform the IC maneuver, which in our case was avoided by keeping the same walking pace of the test for approaching the spirometer. However, we can state that the maneuver is simple to perform and suitable to demonstrate DH in daily clinical practice.32,33
ConclusionsOur study has been able to detect development of DH in COPD male patients even during submaximal exercise such as the 6MWT and more pronouncedly during maximal exercise such as the incremental treadmill CPET. However, these tests showed quite different physiological and perceptual responses, so that analogies for both test are difficult to set. Furthermore, the development of DH cannot be invoked as the only cause of the shortness of breath, especially on a six minute walk test.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare