Chronic obstructive pulmonary disease (COPD) is multi–factorial disorder which results from environmental influences and genetic factors. We aimed to investigate whether methionine sulfoxide reductase A (MSRA) rs10903323 gene polymorphism is associated with COPD development and severity in Serbian adult population.
MethodsThe study included 155 patients with COPD and 134 healthy volunteers. Genotyping was determined performing home-made polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). The difference between the inhibitory activities of normal and oxidized Alpha-1-Antitrypsin (A1AT) against elastase and trypsin was used for determination of Oxidized Alpha-1-Antitrypsin (OxyA1AT) (expressed as % and g/L). Functional activity of A1AT was presented as a specific inhibitor activity to elastase (SIA-Elastase, kU/g).
ResultsFrequencies of the genotypes AA, AG and GG were 80.0%, 20.0%, 0% in COPD patients and 80.5%, 18.5% and 1.5% in the control group, and there was no significant difference in genotype or allele distributions between groups. Serum level of A1AT (g/L) and OxyA1AT was significantly higher in COPD patients than in the control group, but functional activity of A1AT (SIA-Elastase) was significantly lower in COPD patients than in the control group. In COPD group, increased level of OxyA1AT was present in G allele carriers who were smokers relative to G allele carriers who were not smokers. In the smoker group of patients with severe and very severe COPD (GOLD3+4), significant increase in OxyA1AT level was present in G allele carriers compared to AA homozygotes.
ConclusionThese findings suggest that MSRA rs10903323 gene polymorphism is probably not a risk for COPD by itself but could represent a COPD modifier, since minor, G allele, is associated with an increased level of oxidized A1AT, indicating impaired ability of MSRA to repair oxidized A1AT in COPD-smokers, and in severe form of COPD.
Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation that is not fully reversible, which usually progresses, together with an abnormal inflammatory response to noxious particles or gases.1 Genetic predisposition involved in pathogenesis of COPD is the focus of numerous studies. Nowadays, it is clear that the development and progression of this complex disease depends of the multiple genetic and environmental factors. Many studies confirmed that smoking contributes 15% to the variability of lung function and that COPD ultimately develops in 10 to 15% of smokers,2 whilst genetic factors account for more than 40%.3
Alpha-1-antitrypsin (A1AT) is the archetype member of the large protein family of SERPINs (Serine Proteinase Inhibitors), and the major circulating inhibitor of many proteases. It is well known that primary function of A1AT is essential for lung parenchyma, where it protects the alveolar matrix from destruction by neutrophil elastase (NE).4 A1AT inhibits NE at its active site which contains Methionine 358 and 351 which is located in a highly stressed external loop protruding from the molecule.5 Since the methionine residues are vulnerable to oxidation and especially due to its position in A1AT molecule, methionine is easily oxidized which results in forming methionine-sulfoxide, disabling normal antiprotease function of A1AT.6 Beside the hereditary A1AT deficiency, due to mutations in A1AT gene, decreased activity of A1AT due to oxidation of Met3,5,8 by endogenous and exogenous prooxidants, leads to functional deficiency of A1AT with normal serum levels. During inflammatory processes in COPD, many proinflammatory cells, such as neutrophils and macrophages, become activated and liberate reactive oxygen and nitrogen species which may contribute to increased oxidative stress and attack the active centre of A1AT.7 Exogenous sources of oxidants like cigarette smoke and environmental pollution have very important roles in A1AT oxidation.8 It has been documented that methionine-oxidized A1AT isolated from rats was partially restored with addition of Methionine sulfoxide reductase A (MSRA) from the cytosol of human neutrophils in vitro.9 Also, it was shown that MSRA originated from Escherichia coli has the ability to reduce the A1AT which was oxidized by the chloramine-T.10 These suggest that MSRA enzyme activity may be involved in reparation of the damage to proteins that have undergone oxidation, and restoration of their physiological function. MSRA is specific for the reduction of free and protein-based methionine-S-sulfoxide and is the only known enzyme capable of reducing methionine-S-sulfoxide to methionine.11 MSRA was first identified in Escherichia coli, and was later discovered in a large number of organisms with greater level of expression in the kidney and liver than in the heart, lung, brain, skeletal muscle, retina, testis, bone marrow and blood.12,13 In mammals, this enzyme is mainly present in the mitochondrial matrix, but was also found in cytosol and in the nucleus.14
Methionine sulfoxide reductase A (EC 1.8.4.11) is composed of 235 amino acids and is encoded by a 375 kb long gene located on chromosome 8p23.1.15 Data from the literature suggested that the dysfunction of methionine sulfoxide reductases is involved in the pathogenesis of human diseases, such as brain diseases,16 age-associated diseases,17 and vitiligo.18
Since it was revealed that oxidized A1AT can be restored by MSRA, and that the COPD is associated with functional deficiency due to the oxidation of active centre of A1AT, a possible disease modifier could be MSRA gene polymorphism. SNP rs10903323 (A/G) is located in MSRA gene in intron 3. It is assumed that this gene polymorphism may affect the protection of A1AT from prooxidants and influence on its anti-elastase activity. Hence, the question is whether reference A allele or alternative (minor) G allele is associated with higher risk of COPD.
We aimed to explore the association of MSRA rs10903323 gene polymorphism and functional activity of A1AT, as well as the levels of oxidized A1AT with the risk for COPD development and disease severity. Additional goal of this study was to investigate MSRA rs10903323 gene polymorphism for the first-time in the adult population of Serbia, as well as in COPD pathology.
Material and methodsThis study included 155 patients with COPD with a mean age of 64 years, who were recruited from the Clinical for Pulmonary Disease, Clinical Centre of Kragujevac, Republic of Serbia. In the patient group, 64 were declared as current smokers and 67 as current non-smokers. Diagnosis of COPD disease was determined using anamnesis, spirometry tests and physical examination.
Spirometry tests for pulmonary function assessment encompassed measuring FVC (forced vital capacity, % predicted value), FEV1 (forced expiratory volume in 1 second, % of predicted value) and FEV1/FVC ratio. The severity of COPD was defined according to Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD) criterion (Global Initiative for Chronic Obstructive Lung Diseases). Disease severity data according to the GOLD classification were available for 95 patients. Patients with A1AT concentrations below the lower limit of the range (1.1 g/L), indicating a possible congenital A1AT deficiency, were excluded from the study. The control group included 134 healthy volunteers with a mean age of 43 years who were recruited from Health Centre, Belgrade, Serbia. During medical examination it was confirmed that their results of laboratory parameters are in the normal range. All control subjects were without history of pulmonary disease and did not show symptoms of lung disorders currently. The control group consisted of 49 smokers and 83 non-smokers.
Venous blood was sampled from each participant in two tubes, one without anticoagulant and the other coated with EDTA. All of the blood samples and isolated sera were stored at -80˚C until analysis.
All participants gave written consent and completed a questionnaire about age and smoking status. The study protocol was approved by the local Ethic Committee (CCK 11/09/14. No.01/9600) and confirms the ethical guidelines of the Declaration of Helsinki.
MethodsPCR-RFLPA whole blood sample was used for PCR test. The pairs of primers that were used in assay were, For: 5′-GAATAAATAAATGGTGCTGGCCCACACAG-3′, and Rev: 5′- CCAGTCCCTAGATGG
AATCCCACATG-3′ (Thermo Fisher Scientific). PCR mixture was containing 1 µL whole blood sample, 1 pmol of each primer, 1xbuffer with 0.15 mM Mg (Fast Gene), 2 mM dNTP (KAPA Biosystem) and 1 U Taq DNA polymerase (Fast Gene). The PCR conditions were 97˚C for 3 min, 55˚C for 2 min in 3 cycle, and 30 cycles: 94˚C for 30”, 56˚C for 30”, 72˚C for 30” with final elongation at 72˚C for 5 min.
Restriction digestion reaction mixture contained 10 µL of amplified sample, restriction enzyme 1 U Adel (DraIII) (500 U, Thermo Scientific), 1x Buffer G (with BSA) (Thermo Scientific). After incubation over night at 37˚C, digestion products were analysed by electrophoresis on 3 % agarose gel. The distribution of bands was as follows: 190 bp for the uncut product (A allele), and 165 and 25 bp long for cut product (G allele).
Serum level, functional activity and oxidative modification of alpha-1-antitrypsinAssay for Elastase Inhibitory Capacity (EIC) of alpha-1-antitrypsinElastase inhibitor capacity of alpha-1-antitrypsin was determined by modified methods by Bieth et al. 19 and optimized for automatic analyser (Beckman Coulter, Olympus AU400). Stock elastase was prepared by dissolving elastase (Elastase from porcine pancreas min.200 U/mg, SERVA) in a buffer 0.05M TRIS/0.05 NaCl, pH 8.0. Stock substrate was prepared dissolving N-succinyl-L-Alanyl-L-Alanyl-L-Alanyl-p-nitroanilide (STAPNA, SERVA) in 100% dimethyl-sulfoxide (DMSO). 3 µL of diluted stock elastase (1:5) by buffer and 297 µL of buffer were added to serum sample (1:5) and control analyses (40 mg/L albumin). The mixture was incubated for 4 minutes at 37˚C. Immediately after incubation, 25 µL of substrate STAPNA was added to mixture and was reacted with excess of elastase, which was not inhibited by A1AT from serum sample. The change of absorbance due to elastase activity was measured at 410 nm per minute during 5 minutes. EIC in serum sample was calculated using the equitation: EIC (kU/L or mM/L/min)=(ΔA/mincontrol– ΔA/minserum) x f; f=(1/8.8mM) x (337/12) x 5=15.956, where 8.8 mM is molar absorptivity of p-nitroaniline at 410 nm, 337/12 is dilution factor of serum (337 µL is total mixture volume and 12 µL is sample volume) and the factor 5 is initial serum dilution factor.
Assay for Trypsin Inhibitory Capacity (TIC) of alpha-1-antitrypsinTrypsin inhibitory capacity of alpha-1-antitrypsin was assayed by modified methods by Schwert and Takenaka 20 and optimized for automatic analyser (Beckman Coulter, Olympus AU400). As substrate for determining trypsin activity was used N-α-benzoyl-dL-arginine-p-nitroanilide (BAPNA, SIGMA) which was prepared by dissolving in DMSO. 20 µL of bovine trypsin (Trypsin from bovine pancreas ca. ≥10.000 BAEE units/mg protein, SIGMA) previously dissolved in 0.001 M HCl and 280 µL of 0.05M Tris-HCl buffer (pH 8.0) were added to serum sample (1:5) and control analyses (40 g/L albumin). 25 µL of substrate was added after an incubation period of 4 minutes at 37˚C, after which started the lag phase lasting for 1 minute. Then the change in absorbance was measured for 5 minutes per minute at wavelength 410 nm.TIC in serum sample was calculated using the equation: TIC (kU/L or mM/L/min)=(ΔA/mincontrol– ΔA/minserum) x f; f=(1/8.8mM) x (329/4), where 8.8 is molar absorbance of p-nitroaniline at 410 nm, 329/4 is dilution factor of serum and the factor 5 is initial serum dilution factor.
The serum level of A1AT (g/L) was determined by immunoturbidimetric method using Siemens ADVIA R Chemistry Analyzer and Alpha-1-antitrypsin Reagent. Specific inhibitory activity of A1AT towards elastase (SIA-Elastase) were calculated using equations: SIA-Elastase (kU/g)=EIC(kU/L)/A1AT(g/L). Level of oxidized A1AT (OxyA1AT, %) was calculated using equation OxyA1AT (%)=[1−(1.27/TICsample/EICsample) x 100], where the value 1.27 represents the TICreduced/EICreduced ratio of the fully reduced A1AT by mercaptoethanol, while the value TICsample/EICsample in serum sample represents the oxidized ratio.21 Calculation of OxyA1AT (g/L) was done using equation: OxyA1AT (g/L)=[OxyA1AT (%) x A1AT (g/L)]/100.
Statistical analysisThe one-sample Kolmogorov-Smirnov test was used to estimate normality of the distribution of variables. Differences between genotypes and allele frequencies of methionine sulfoxide reductase A rs10903323 polymorphism were evaluated using χ2-test. Deviations of genotypes distributions from Hardy–Weinberg equilibrium were assessed by χ2-test for each cohort or Fisher's exact test (if cases <5). Student t-test for independent samples was used for comparison of continuous variables. Three-way ANOVA and Sidak post hoc test were used to analyse differences in continuous variables according to genotype, smoking status, and disease development or severity. The p value <0.05 was regarded as statistically significant. Statistical analysis was done using SPSS 20.0 software.
ResultsThe demographic data, clinical characteristics, functional activity and oxidative modification of A1AT in all study participants are presented in Table 1. Kolmogorov-Smirnov test showed normal distribution of quantitative variables (p>0.05). COPD patients were significantly older and had a lower body mass index (BMI) than healthy subjects (p < 0.001, p = 0.007 respectively). Serum level of A1AT (g/L) and OxyA1AT (expressed as % of total A1AT and g/L) was significantly higher in COPD patients than in the control group (p < 0.001, p = 0.033, p < 0.001, respectively). However, activity of A1AT, measured by SIA-Elastase, was significantly lower in COPD patients than in the control group (p < 0.001).
Demographic data, clinical characteristics, parameters of functional activity and oxidative modification of A1AT in COPD-patient and control groups.
Values for the quantitative variables are presented as the mean ± standard deviation; smoking status, FEV1/FVC ratio and COPD stage are presented as percentages; FEV1 and FVC are expressed as % of predicted value a p value <0.05.
Abbreviations: A1AT, alpha-1-antitrypsin; COPD, chronic obstructive pulmonary disease; BMI, body mass index; SIA-elastase, specific inhibitory activity of A1AT towards elastase; OxyA1AT, oxidized alpha-1-antitrypsin; FEV1(%), forced expiratory volume in 1 s; FVC (%), forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Observed frequencies of genotypes and alleles of MSRA rs10903323 in COPD patient and the control group are presented in Table 2. Frequencies of MSRA genotypes in both populations were consistent with Hardy-Weinberg equilibrium (COPD group: χ2=1.91, p = 0.380; control group: χ2=0.25, p = 0.880). A significant difference in genotype distribution between COPD patients and controls (p = 0.289) was not found in this study. Frequencies of the genotypes AA, AG and GG were 80.0 %, 20.0 %, 0 % in COPD patients and 80.5 %, 18.5 % and 1.5 % in the control group. Also, there was no significant difference between COPD and the control group when the frequencies of MSRA rs10903323 minor allele carriers (AG and GG genotype) were compared to homozygote AA carriers (p = 0.889).
Frequencies of genotypes and alleles of MSRA rs10903323 in COPD-patient group and in control groups.
| MSRA rs10903323 | COPD-patients (n=155) | Control (n=134) | P* |
|---|---|---|---|
| n (%) | n (%) | ||
| Genotype | |||
| AA | 124 (80.0) | 108 (80.5) | 0.887 |
| AG | 31 (20.0) | 24 (18.0) | 0.654 |
| GG | 0 | 2 (1.5) | 0.214 |
| AG+GG | 31 (20.0) | 26 (19.5) | 0.887 |
| Allele | |||
| A | 279 (90.0) | 240 (89.5) | 0.862 |
| G | 31 (10.0) | 28 (10.5) | 0.862 |
| H-W, χ2 (P⁎⁎) | 1.91 (0.380) | 0.25 (0.880) |
Abbreviations: MSRA, methionine sulfoxide reductase A; COPD, chronic obstructive pulmonary disease; H-W, Hardy-Weinberg equilibrium.
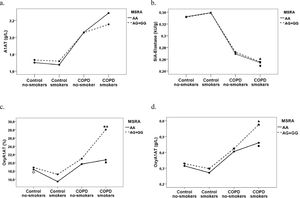
A three-way ANOVA test was conducted to explore the influence of MSRA rs10903323 gene polymorphism, presence of disease and smoking status on functional activity of A1AT and OxyA1AT in COPD patient and the control group (Fig. 1.). The combined influence between MSRA rs10903323 gene polymorphism, smoking status and presence/absence of COPD on the OxyA1AT (%) was not statistically significant, F (1.209) = 0.803, p = 0.371. SIA-Elastase was significantly lower in patient-smokers than in control-smokers in both AA homozygotes (p < 0.001), and in G allele carriers (p = 0.027) (Fig. 1 a.).
The influence of MSRA rs10903323 gene polymorphism on parameters of functional activity of A1AT and level of oxidized A1AT in COPD patients and the control group classified according to smoking status as smokers and non-smokers: a. concentration of A1AT; b. SIA-Elastase (kU/g); c. OxyA1T (%) and d. OxyA1AT (g/L) (Tested by Sidac post hoc test); ■ difference in AA homozygous patients-smokers in relation to control-smokers; ▲ difference in G allele carriers patients-smokers in relation to control-smokers; ★ difference in G allele carriers patients-smokers in relation to AA homozygous patients-smokers; ○difference in controls non-smokers in relation to control-smokers
Abbreviations: A1AT, alpha-1-antitrypsin; MSRA, Methionine sulfoxide reductase A; COPD, chronic obstructive pulmonary disease; OxyA1AT, oxidized alpha-1-antitrypsine; SIA-elastase, specific inhibitory activity of A1AT towards elastase.
Using the Sidak post hoc test revealed that COPD-smokers who are G allele carriers of MSRA rs10903323 had significantly higher OxyA1AT (%) than AA homozygotes (p = 0.028, partial eta squared=0.023) (Fig. 1 c.). In group of smokers, levels of OxyA1AT (%) and Oxy A1AT (g/L) were significantly increased in patients relative to controls in AA homozygous (p = 0.001, p < 0.001 respectively), as well as in G allele carriers (p = 0.008, p = 0.002 respectively) (Fig. 1.. c., d.). In the control group, AA homozygous non-smokers showed significantly higher level of OxyA1AT (%) than smokers (p = 0.043) (Fig. 1 c.). In the COPD group, increased level of Oxy A1AT (both expressed as % and g/L) was present in G alleles carriers who were smokers relative to G alleles carriers who were non-smokers, with probability close to the level of significance (p = 0.056, p = 0.056 respectively) (Fig. 1 c., d.).
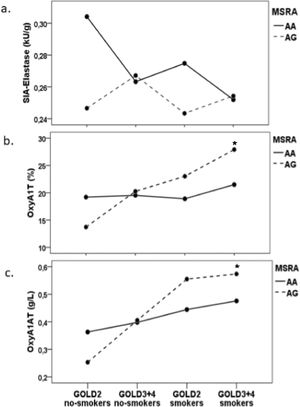
A three-way ANOVA test was conducted to explore the influence of MSRA rs10903323 polymorphism, COPD severity and smoking status on functional activity of A1AT and OxyA1AT in COPD patient group (Fig. 2.). The combined influence of MSRA rs10903323 polymorphism, disease severity and smoking status on the all of three investigated parameters was not found (for SIA-Elastase (F (3.76) = 0.471, p = 0.703; for OxyA1AT (%) (F (3.76)=0.997, p = 0.399; for OxyA1AT (g/L) (F (3.76)=0.714, p = 0.547)). The difference in the functional activity of the A1AT relative to the MSRA rs10903323 genotype can be seen only in the moderate stage of disease (GOLD2 group) where SIA-Elastase was lower in G allele carriers than in AA homozygotes (Fig. 2 a.), but without statistical significance. Using the Sidak post hoc test revealed tendency for smokers with severe and very severe COPD (GOLD3+4) who are G allele carriers of MSRA rs10903323 to have higher OxyA1AT (%) than AA homozygotes (p = 0.086) (Fig. 2 b.). Patients with severe and very severe disease who were smokers and carriers of G allele, had significantly increased levels of OxyA1AT (%, g/L), compared to AA homozygotes (using independent sample t-test, p < 0.001, p = 0.041, respectively).
The influence of MSRA rs10903323 gene polymorphism on parameters of functional activity of A1AT and level of oxidized A1AT in COPD patients classified in two groups according to disease severity (GOLD2 and GOLD 3+4) and smoking status (smokers and non-smokers): a. SIA-Elastase (kU/g); b. OxyA1T (%) and c. OxyA1AT (g/L); ★ difference in smokers patients (GOLD3+4) G allele carriers in relation to AA homozygous.
Abbreviations: MSRA, Methionine sulfoxide reductase A; OxyA1AT, oxidized alpha-1-antitrypsin; SIA-elastase, specific inhibitory activity of A1AT towards elastase; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
We have investigated the association of MSRA rs10903323 gene polymorphism with COPD pathology, with emphasis on its impact on A1AT activity. The association between MSRA and COPD may be explained by the fact that MSRA can partially repair the oxidized methionine in active centre of A1AT,22 and restore its anti-elastase function. The association of single nucleotide polymorphisms (SNPs) of the MSRA gene with human diseases has not been intensively investigated in worldwide populations. According to available data, this is the first study which investigated the MSRA rs10903323 gene polymorphism in the Serbian population. To the best of our knowledge, MSRA rs10903323 gene polymorphism was investigated for the first time in COPD pathology. The results obtained for our population (AA 80 %, AG 18.5 % and GG 1.5 %) are in concordance with available data for European general population (AA 78.3 %, AG 20.5 % and GG 1.2 %),23 regarding both genotype and allele distribution. Distribution of MSRA rs10903323 genotypes varies significantly between different populations. For example, frequencies of AA, AG and GG genotypes in population of East Asia are 15.1 %, 49.2 %, 35.7 %, and in population of America are 50.7 %, 32.0 %, 17.3 %.23 We did not find any difference in distribution of MSRA rs10903323 genotypes (AA, AG and GG) and alleles between COPD patients and the control group, indicating that this polymorphism does not present a risk factor for development of COPD, but can rather act as a modifier of disease progression or severity. Also, there was no difference in frequency for genotype AA and genotype AG+GG between the two investigated groups.
Considering that COPD is an inflammatory disease of the airways, mainly associated with cigarette smoke exposure, our result of elevated A1AT concentrations in patients compared to healthy subjects was expected because A1AT is a positive acute phase protein. High concentration of A1AT is a physiological response to an excessive liberation of NE by neutrophils, which protects an organism from the uncontrolled proteolysis of host tissues.24 We have found that COPD patients had a higher level of OxyA1AT (expressed as % and g/L) than the control group. This result indicates that higher A1AT concentration in patients with COPD is not associated with the better anti-elastase activity because an elevated level of oxidative modified A1AT causes the decrease of its functional activity. This assumption is further strengthened by the fact that we have found reduced A1AT effective anti-elastase activity in patients compared to healthy participants, through the decreased SIA-Elastase (Table 1.).
Cigarette smoking (CS) has traditionally been considered a major risk factor for developing COPD, due to the harmful effect of numerous prooxidants on the lung function. Many studies investigated gene expression in relation to smoking behaviour. Gene expression levels may be up-regulated or down-regulated as a consequence of smoking and also may be associated with the number of cigarettes per day.25 There is limited data about the influence of CS on MSRA expression. One study showed an increase in MSRA expression after CS exposure in HaCaT cells.26 The main result of our study was identification of elevated OxyA1AT (%) in patient-smokers group of G allele carriers in relation to patient-smokers with AA genotype (Fig. 1c.). Additionally, among the G allele carriers, COPD patients who were smokers had increased levels of OxyA1AT (%, g/L) in relation to patients who were non-smokers (Fig. 1c., d.). This suggests that the presence of G allele in current smokers may contribute to an increased risk for COPD, at least regarding the OxyA1AT level which is thought to participate in the pathogenesis of the disease.27 These results indicate the importance of smoking cessation, in patients with diagnosed disease, and especially in carriers of the of MSRA rs10903323 G allele. An increased level in OxyA1AT level was also observed in the group of smokers who were AG heterozygotes, and who had a more severe form of the disease (GOLD 3+4) compared to patients with the same disease severity, but who were AA homozygotes (Fig. 2 b., c.). This study revealed a potential modifier gene that leads to inadequate A1AT repair in smokers, resulting in an increase in oxidative modified A1AT. This modifier is a MSRA rs10903323 G allele and it is associated with a higher risk of a more severe form of COPD. This polymorphism was shown to be connected with several disorders characterized by chronic inflammation and increased oxidative stress, similar to COPD (rheumatoid arthritis (RA), coronary artery disease). Two studies found association of this polymorphism with RA .28,29 In genome-wide pathway analysis Martín et al concluded that MSRA rs10903323 gene polymorphism was related to increased oxidative stress and pathogenesis of RA in a Spanish population.28 Similar data was obtained in a Chinese population,29 where MSRA rs10903323 GA genotype was associated with rheumatoid arthritis development, especially among older male patients, and CRP-positive patients. The MSRA rs10903323 minor allele G was additionally identified as the risk for development of ischaemic heart disease, observed in patients with RA, in Spanish cohort.30 Gu et al demonstrated that subjects with heterozygous GA genotype were at a significantly increased risk for coronary artery disease in the Chinese population.31
Considering that COPD, rheumatoid arthritis and cardiovascular diseases share common causes which involve inflammation, we can suggest that the presence of the MSRA rs10903323 G allele is a risk for COPD due to the reduced antioxidant functions of MSRA. In COPD pathology we showed specificity that G allele is associated with increased oxidative modified A1AT, of which the main physiological function is protection of lower respiratory tract. In this context, the determination of oxidized A1AT, which is relatively simple and inexpensive, may be useful for identification of particularly vulnerable individuals with COPD, namely smokers and carriers of MSRA rs10903323 G allele. Also, the potential clinical significance of the MSRA rs10903323 polymorphism could be for patients with hereditary A1AT deficiency which is associated with decreased plasma level of this antiprotease. In these patients, detection of the MSRA rs10903323 G allele, in addition to hereditary A1AT deficiency, could be considered an additional risk for developing lung diseases (such as emphysema). For this group of susceptible patients, strict preventative measures against the exposure to tobacco smoke or other air pollutants and pro-oxidants would have to be carried out throughout their lifetime.
There were several limitations to our study. As the groups tested were relatively small, these findings need to be confirmed by additional studies with a larger sample size, preferably in different populations. There are several more polymorphisms in MSRA gene and some of them could also be important for enzyme activity regarding restoration of A1AT function. The information about their presence would help to identify the full extent to which MSRA affects COPD phenotype. It would also be beneficial to directly measure the expression and/or the activity of MSRA gene/enzyme in participant samples, in order to confirm that the presence of the analysed variants has a direct impact on MSRA ability to perform its antioxidizing function effectively. This data is not available in the current literature.
ConclusionsOur study is the first one analysing the association of MSRA rs10903323 gene polymorphism with the risk of development and severity of COPD. The assumption is that MSRA G allele is associated with decreased ability of MSRA to reduce oxidized A1AT in patient-smokers, resulting in an impaired antioxidant protection against prooxidants from tobacco smoke. Our results might be useful to clarify the contribution of MSRA rs10903323 gene polymorphism in individual predisposition to COPD and its role in disease pathogenesis.
FundingThis research was funded by the Ministry of Education, Science and Technological Development, Republic of Serbia through Grant Agreement with University of Belgrade-Faculty of Pharmacy No: 451-03-9/2021-14/200161 and Grant Agreement with IMGGE, University of Belgrade No. 451-03-9/2021-14/200042.
Authors would like to thank Mr. Andrew Carruthers (Toronto, Canada) for the proofreading and editing.