There is a great heterogeneity in the prevalence of Chronic Obstructive Pulmonary Disease (COPD) across the world. The Burden of Obstructive Lung Disease (BOLD) initiative was started to measure the prevalence of COPD in a standardized way. We aimed to estimate the prevalence of COPD in Portuguese adults aged 40 years or older of a target population of 2,700,000 in the Lisbon region, in accordance with BOLD protocol.
MethodsA stratified, multi-stage random sampling procedure was used which included 12 districts. The survey included a questionnaire with information on risk factors for COPD and reported respiratory disease and a post-bronchodilator spirometry performed at survey centres.
ResultsFor the 710 participants with questionnaires and acceptable spirometry, the overall weighted prevalence of GOLD stage I+ COPD was 14.2% (95% C.I. 11.1, 18.1), and stage II+ was 7.3% (95% C.I. 4.7, 11.3). Unweighted prevalence was 20.2% (95% C.I.17.4, 23.3) for stage I+ and 9.5% (95% C.I. 7.6, 11.9) for stage II+. Prevalence of COPD in GOLD stage II+ increased with age and was higher in men. The prevalence of GOLD stage I+ COPD was 9.2% (95% C.I. 5.9, 14.0) in never smokers versus 27.4% (95% C.I. 18.5, 38.5) in those who had smoked ≥20 pack-years. The agreement between previous doctor diagnosis and spirometric diagnosis was low, with 86.8% of underdiagnosed individuals.
ConclusionsThe 14.2% of COPD estimated prevalence indicates that COPD is a common disease in the Lisbon region. In addition, a large proportion of underdiagnosed disease was detected. The high prevalence of COPD with a high level of underdiagnosis, points to the need of raising awareness of COPD among health professionals, and requires more use of spirometry in the primary care setting.
A prevalência da doença pulmonar obstrutiva crónica (DPOC) apresenta valores muito heterogéneos em todo o mundo. A iniciativa Burden of Obstructive Lung Disease (BOLD) foi desenvolvida para que a prevalência da DPOC possa ser avaliada com metodologia normalizada. O objetivo deste estudo foi estimar a prevalência da DPOC em adultos com 40 ou mais anos numa população alvo de 2700000 habitantes na região de Lisboa, de acordo com o protocolo BOLD.
MétodosA amostra foi estratificada de forma aleatória multifaseada selecionando-se 12 freguesias. O inquérito compreendia um questionário com informação sobre fatores de risco para a DPOC e doença respiratória autoreportada; adicionalmente, foi efetuada espirometria com prova de broncodilatação.
ResultadosForam incluídos 710 participantes com questionário e espirometria aceitáveis. A prevalência estimada da DPOC na população no estádio GOLD I+ foi de 14,2% (IC 95%: 11,1; 18,1) e de 7,3% no estádio ii+ (IC 95%: 4,7; 11,3). A prevalência não ajustada foi de 20,2% (IC 95%: 17,4; 23,3) no estádio i+ e de 9,5% (IC 95%: 7,6; 11,9) no estádio ii+. A prevalência da DPOC no estádio GOLD II+ aumentou com a idade, sendo mais elevada no sexo masculino. A prevalência estimada da DPOC no estádio GOLD I+ foi de 9,2% (IC 95%: 5,9; 14,0) nos não fumadores versus 27,4% (IC 95%: 18,5; 38,5) nos fumadores com carga tabágica de ≥ 20 Unidades Maço Ano. Detetou-se uma fraca concordância entre a referência a diagnóstico médico prévio e o diagnóstico espirométrico, com 86,8% de subdiagnósticos.
ConclusõesO achado de uma prevalência estimada da DPOC de 14,2% sugere que esta é uma doença comum na região de Lisboa, contudo com uma elevada proporção de subdiagnósticos. Estes dados apontam para a necessidade de aumentar o grau de conhecimento dos profissionais de saúde sobre a DPOC, bem como a necessidade de maior utilização da espirometria nos cuidados de saúde primários.
Chronic Obstructive Pulmonary Disease (COPD) was the sixth-most common cause of death worldwide in 1990, but is projected to become the third-most common cause by the year 20301; it is already ranked fourth in developed countries,2–5 with approximately 2.75 million deaths per year, or 4.8% of all deaths.3
The prevalence of COPD in the general population across all ages rises steeply to above 10% amongst people who are aged over 40. The prevalence increases considerably with age.2
In Portugal, the previously reported prevalence was 5.34%,6 based on a study of 2002. However, this study had some methodological limitations related not only to the age range of the individuals studied (35–69 years old), but also to the criteria used for airway obstruction definition (pre-bronchodilator fixed ratio criteria) due to the absence of a bronchodilator test. In fact, epidemiological studies should not exclude older individuals because life expectancy in Portugal, is higher than 70 years old (respectively 76 and 82 years for males and females).7
COPD prevalence across the world varies considerably, due to differences in methodologies, sampling and diagnostic criteria for COPD, and also to differences in patterns of cigarette smoking.
Accurate prevalence studies are needed to guide future projections of the burden of this disease in each country, and to assist public health officials in the planning to meet the growing demand for health care resources.8
The Burden of Obstructive Lung Disease (BOLD) Initiative developed standardized methods for estimating of COPD prevalence. These methods can be used in countries at all levels of development to measure the worldwide prevalence of COPD and its risk factors in adults aged 40 years and older, and to investigate variation in prevalence across countries by age, gender, and smoking status.8 Overall 20 countries have completed their study participation and other sites are in progress.9
The aim of our study was to estimate the prevalence of COPD based on a representative sample of non-institutionalized adults aged 40 years and over, carried out in Lisbon, Portugal, using standardized post-bronchodilator (post-BD) spirometry, as per the BOLD protocols.
Material and methodsWe conducted a cross-sectional population survey following BOLD protocol, as described in detail elsewhere.10 Study fieldworkers were trained and certified in study methodology.
Sample sizeA minimum sample size of 600 individuals was considered to provide an acceptable level of precision for prevalence estimates, assuming simple random sampling.10
Study populationThe survey was conducted among the residents of Lisbon region, whose population represents nearly 27% of the total Portuguese inhabitants, according to the 2007 census. The survey area was divided into two broad zones which are distinct regarding the demographic structure, socio-economic status, population density and environmental stress: the inner city zone, with about 800,000 residents, and the suburbs with about 1,900,000 residents.
We used a stratified, multi-stage random sampling procedure. In the first sampling stage, 8 suburban and 4 inner-city districts were randomly selected from a total of 173 suburban and 53 inner-city districts of Lisbon. In the second sampling stage, households from the selected districts were randomly selected using a phone list that covered over 90% of the inhabitants of the districts selected. In the third sampling stage, individuals from the households selected were invited to participate if they were aged 40+. There was also a Minimal Data/Refusal questionnaire for participants who were not willing to participate in the full protocol (non responders). A professional survey-specialized phone-call centre was contracted to make contacts and invite participation. Data were collected between 16th of June and 7th of November 2008.
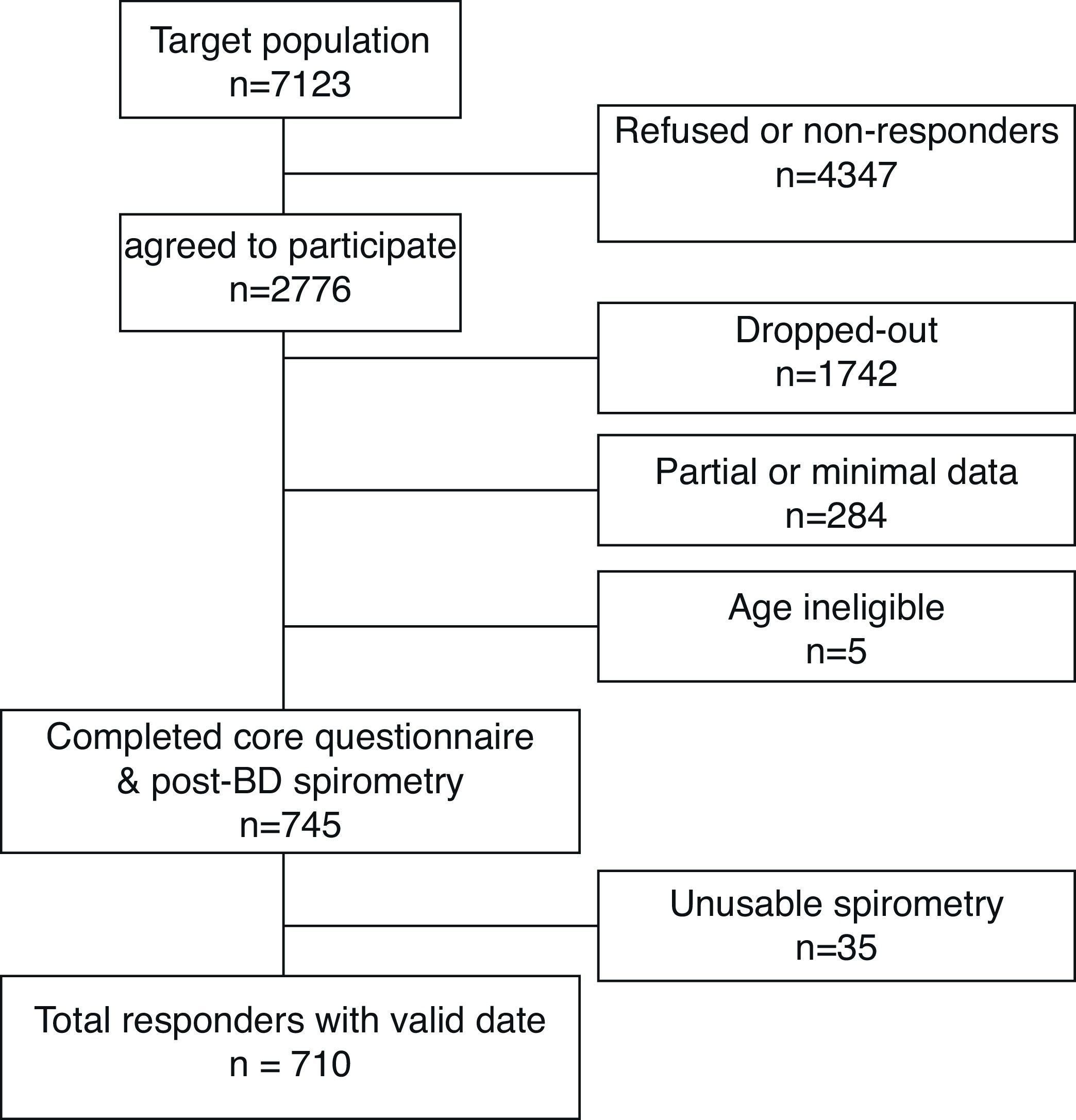
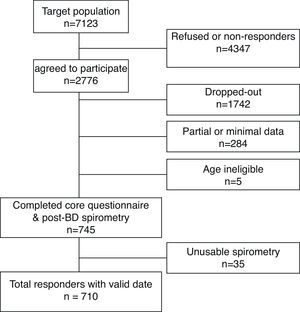
Of the 7123 individuals, whom we attempted to contact, 2776 agreed (over the phone) to take part in the survey. From those, 745 (27%) completed the protocol (core questionnaire plus post-BD spirometry), but 35 participants did not meet the American Thoracic Society (ATS) spirometry quality control criteria. Therefore, 710 responders (331 men and 379 women) constituted the final sample for this analysis (Fig. 1).
Written informed consent was obtained from each participant and ethics approval was granted by Ethics Committee of Hospital Pulido Valente and by the Portuguese Data Protection Authority.
SpirometryAt survey centres located in each county, pre and post-BD spirometry tests were performed according to the ATS guidelines11 by trained and certified technicians using the nddEasyOne™ Spirometer (ndd Medizintechnik; Zurich, Switzerland). At least three technically acceptable manoeuvres were performed, in a seated position, to obtain a minimum of two reproducible spirometry tests, with variability less than 200mL, for both the forced expiratory volume in one second (FEV1) and forced vital capacity (FVC). Two puffs of salbutamol (200μg) were administered with a metered dose inhaler connected to a spacer (15cm length/2.1cm diameter) and 20min later the test (post-BD spirometry) was repeated. Spirometry tests were defined as acceptable, if they were free from artefacts, sudden stops, and back-extrapolated volumes greater than 5.0% of FVC or 150mL, whichever was greater.11
Spirometry data were sent electronically to the Pulmonary Function Quality Control Centre (Co-ordinating Centre in UK) where each spirometry was reviewed and graded according to its quality, based on criteria from the BOLD project.11 Study technicians were continuously monitored and if a technician's quality-score dropped below a pre-set level, he/she had to stop testing and be re-trained and re-certified.
Questionnaire dataAll participants answered BOLD questionnaires with demographic and clinical data, including doctor-diagnosed respiratory conditions.10
The original BOLD questionnaires were translated from English to Portuguese and then back-translated to assure accuracy and conceptual equivalence. Questionnaires were obtained by face-to-face interview with trained and certified staff. A core questionnaire was completed for all individuals considered as responders. A minimal data/refusal questionnaire was collected from the non-responders group. Non-responders were eligible individuals who missed the core questionnaire and/or post-BD spirometry. By contrast responders completed both items. All the questionnaires were revised for completeness, accuracy and consistency within 48hours of the interview by a team of seven certified physicians. All data were sent and quality controlled by the BOLD Co-ordinating Centre in UK.
DefinitionsCOPD was defined based on post-BD spirometry. In accordance with the Global Initiative for COPD (GOLD) guidelines,12 a not fully reversible airway obstruction was defined, as a post-BD FEV1/FVC<0.7. This definition was also used for COPD stage I+ in the analysis. Severity of COPD was defined as stage II+ if post-BD FEV1<80% predicted, as stage III+ if post-BD FEV1<50% predicted and stage IV if post-BD FEV1<30%. Percent predicted values13 for Caucasian men and women were calculated using the National Health and Nutrition Examination Survey (NHANES) III reference equations.13
COPD diagnosis was considered based on the post-BD lung function criteria without requiring the presence of symptoms or documented exposure to a known causative agent, as per BOLD protocol.8–10
COPD doctor's diagnosis (dx) was defined as the self-reported physician's diagnosis of emphysema, chronic bronchitis or COPD.
Subjects were classified as current smokers, ever smokers or never smokers. Ever-smokers (including former and current smokers) were defined as individuals who smoke more than 20 packs of cigarettes in a lifetime or more than 1 cigarette per day during one year. The number of pack-years of cigarette smoking was defined as the average number of cigarettes smoked per day divided by 20 (i.e. packs per day) times the duration of smoking in years. Because of the low occurrence of stage IV COPD in the population samples, stages III and IV were combined in this paper.
Statistical analysisThis analysis included 710 Portuguese participants who completed the core questionnaire and who had acceptable post-BD spirometry measures. Results are presented stratified by gender, age-groups, pack-years smoked and as totals.
Prevalence estimates were calculated for the overall Lisbon population, as well as for subgroups defined by gender and either age or pack-years of cigarette smoking. Because the distribution of participants in the responders’ data differed slightly from that for Lisbon population, the data were weighted according to age and gender group, so that there would be a better match of the resulting prevalence estimates with those of Lisbon as a whole. In the weighting process each subject had a weight attached to him, where the weight corresponds to the number of people this subject represents in the population. The weighting was used in order to overcome limitations of sample survey, such as differential non-response rates, or under coverage of some sub-populations.
Weighted population-based estimates of COPD prevalence and their respective 95% confidence intervals (C.I.) were computed using survey data methods in STATA (STATA Corporation, College Station, TX, USA). These calculations were made to assure that the estimated prevalence and the respective 95% C.I. properly reflected the sampling design.
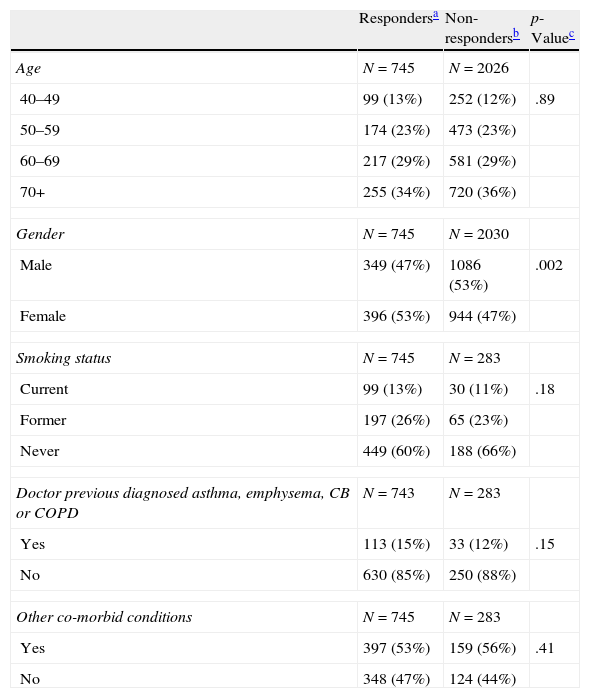
ResultsTable 1 shows that there were no differences between responders and non-responders who completed minimal data questionnaire, except for gender, where non-responders were predominantly male.
Comparison of responders and non-responders for BOLD Portugal.*
| Respondersa | Non-respondersb | p-Valuec | |
| Age | N=745 | N=2026 | |
| 40–49 | 99 (13%) | 252 (12%) | .89 |
| 50–59 | 174 (23%) | 473 (23%) | |
| 60–69 | 217 (29%) | 581 (29%) | |
| 70+ | 255 (34%) | 720 (36%) | |
| Gender | N=745 | N=2030 | |
| Male | 349 (47%) | 1086 (53%) | .002 |
| Female | 396 (53%) | 944 (47%) | |
| Smoking status | N=745 | N=283 | |
| Current | 99 (13%) | 30 (11%) | .18 |
| Former | 197 (26%) | 65 (23%) | |
| Never | 449 (60%) | 188 (66%) | |
| Doctor previous diagnosed asthma, emphysema, CB or COPD | N=743 | N=283 | |
| Yes | 113 (15%) | 33 (12%) | .15 |
| No | 630 (85%) | 250 (88%) | |
| Other co-morbid conditions | N=745 | N=283 | |
| Yes | 397 (53%) | 159 (56%) | .41 |
| No | 348 (47%) | 124 (44%) | |
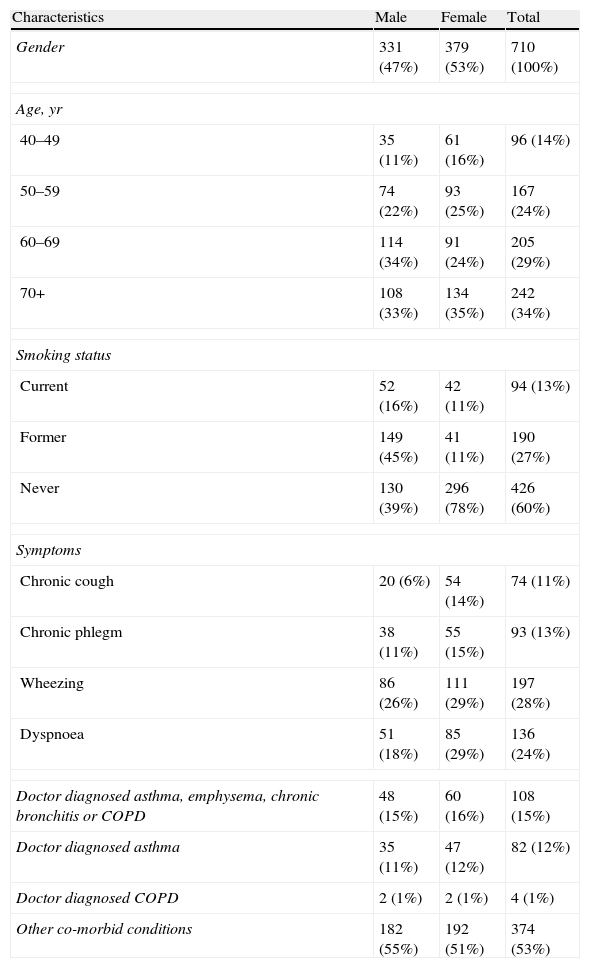
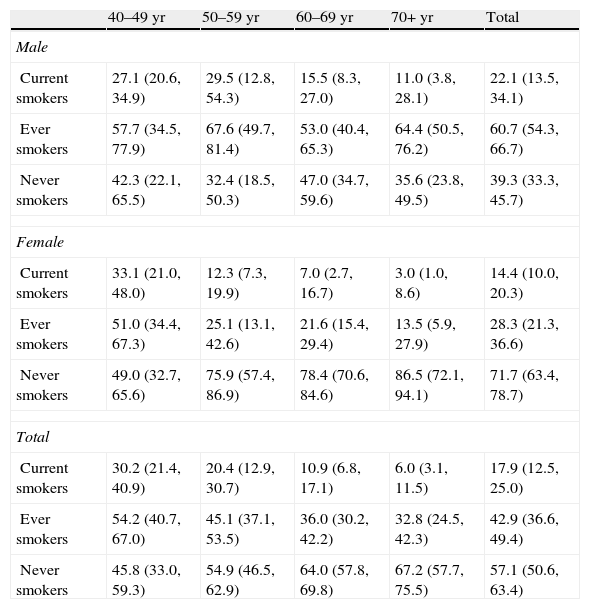
Table 2 summarizes the main characteristics of the participants’ final sample. More than half of the subjects (63.0%) were 60 years or older and 53.4% were female. Current or former smoking was more frequent in men, while never smoking was more prevalent in women. Table 3 presents weighted population estimate of smoking habits by age and gender (unweighted data are shown in Table 3a of the electronic appendix). The prevalence of total current smokers was 17.9% (95% C.I. 12.5, 25.0). The highest current smoking prevalence occurred in the female 40–49 year age-group (33.1%, 95% C.I. 21.0, 48.0), decreasing with age. Among men, the highest prevalence was in the 50–59 year age-group. Ever smoking showed the highest prevalence in men aged 70+ years old.
Characteristics of the study sample.*
| Characteristics | Male | Female | Total |
| Gender | 331 (47%) | 379 (53%) | 710 (100%) |
| Age, yr | |||
| 40–49 | 35 (11%) | 61 (16%) | 96 (14%) |
| 50–59 | 74 (22%) | 93 (25%) | 167 (24%) |
| 60–69 | 114 (34%) | 91 (24%) | 205 (29%) |
| 70+ | 108 (33%) | 134 (35%) | 242 (34%) |
| Smoking status | |||
| Current | 52 (16%) | 42 (11%) | 94 (13%) |
| Former | 149 (45%) | 41 (11%) | 190 (27%) |
| Never | 130 (39%) | 296 (78%) | 426 (60%) |
| Symptoms | |||
| Chronic cough | 20 (6%) | 54 (14%) | 74 (11%) |
| Chronic phlegm | 38 (11%) | 55 (15%) | 93 (13%) |
| Wheezing | 86 (26%) | 111 (29%) | 197 (28%) |
| Dyspnoea | 51 (18%) | 85 (29%) | 136 (24%) |
| Doctor diagnosed asthma, emphysema, chronic bronchitis or COPD | 48 (15%) | 60 (16%) | 108 (15%) |
| Doctor diagnosed asthma | 35 (11%) | 47 (12%) | 82 (12%) |
| Doctor diagnosed COPD | 2 (1%) | 2 (1%) | 4 (1%) |
| Other co-morbid conditions | 182 (55%) | 192 (51%) | 374 (53%) |
Estimated-population prevalence of smoking habits by age and gender.*
| 40–49 yr | 50–59 yr | 60–69 yr | 70+ yr | Total | |
| Male | |||||
| Current smokers | 27.1 (20.6, 34.9) | 29.5 (12.8, 54.3) | 15.5 (8.3, 27.0) | 11.0 (3.8, 28.1) | 22.1 (13.5, 34.1) |
| Ever smokers | 57.7 (34.5, 77.9) | 67.6 (49.7, 81.4) | 53.0 (40.4, 65.3) | 64.4 (50.5, 76.2) | 60.7 (54.3, 66.7) |
| Never smokers | 42.3 (22.1, 65.5) | 32.4 (18.5, 50.3) | 47.0 (34.7, 59.6) | 35.6 (23.8, 49.5) | 39.3 (33.3, 45.7) |
| Female | |||||
| Current smokers | 33.1 (21.0, 48.0) | 12.3 (7.3, 19.9) | 7.0 (2.7, 16.7) | 3.0 (1.0, 8.6) | 14.4 (10.0, 20.3) |
| Ever smokers | 51.0 (34.4, 67.3) | 25.1 (13.1, 42.6) | 21.6 (15.4, 29.4) | 13.5 (5.9, 27.9) | 28.3 (21.3, 36.6) |
| Never smokers | 49.0 (32.7, 65.6) | 75.9 (57.4, 86.9) | 78.4 (70.6, 84.6) | 86.5 (72.1, 94.1) | 71.7 (63.4, 78.7) |
| Total | |||||
| Current smokers | 30.2 (21.4, 40.9) | 20.4 (12.9, 30.7) | 10.9 (6.8, 17.1) | 6.0 (3.1, 11.5) | 17.9 (12.5, 25.0) |
| Ever smokers | 54.2 (40.7, 67.0) | 45.1 (37.1, 53.5) | 36.0 (30.2, 42.2) | 32.8 (24.5, 42.3) | 42.9 (36.6, 49.4) |
| Never smokers | 45.8 (33.0, 59.3) | 54.9 (46.5, 62.9) | 64.0 (57.8, 69.8) | 67.2 (57.7, 75.5) | 57.1 (50.6, 63.4) |
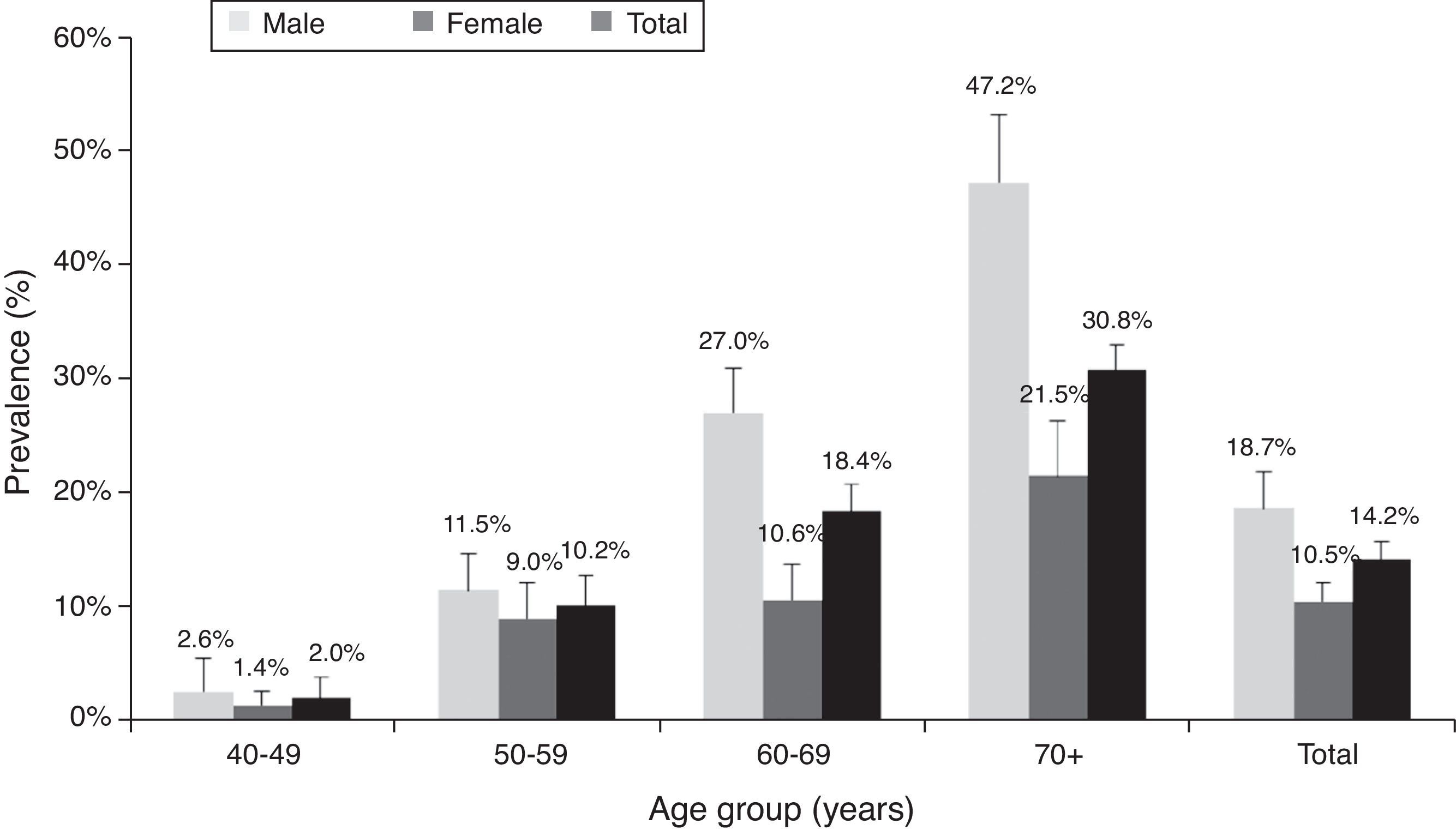
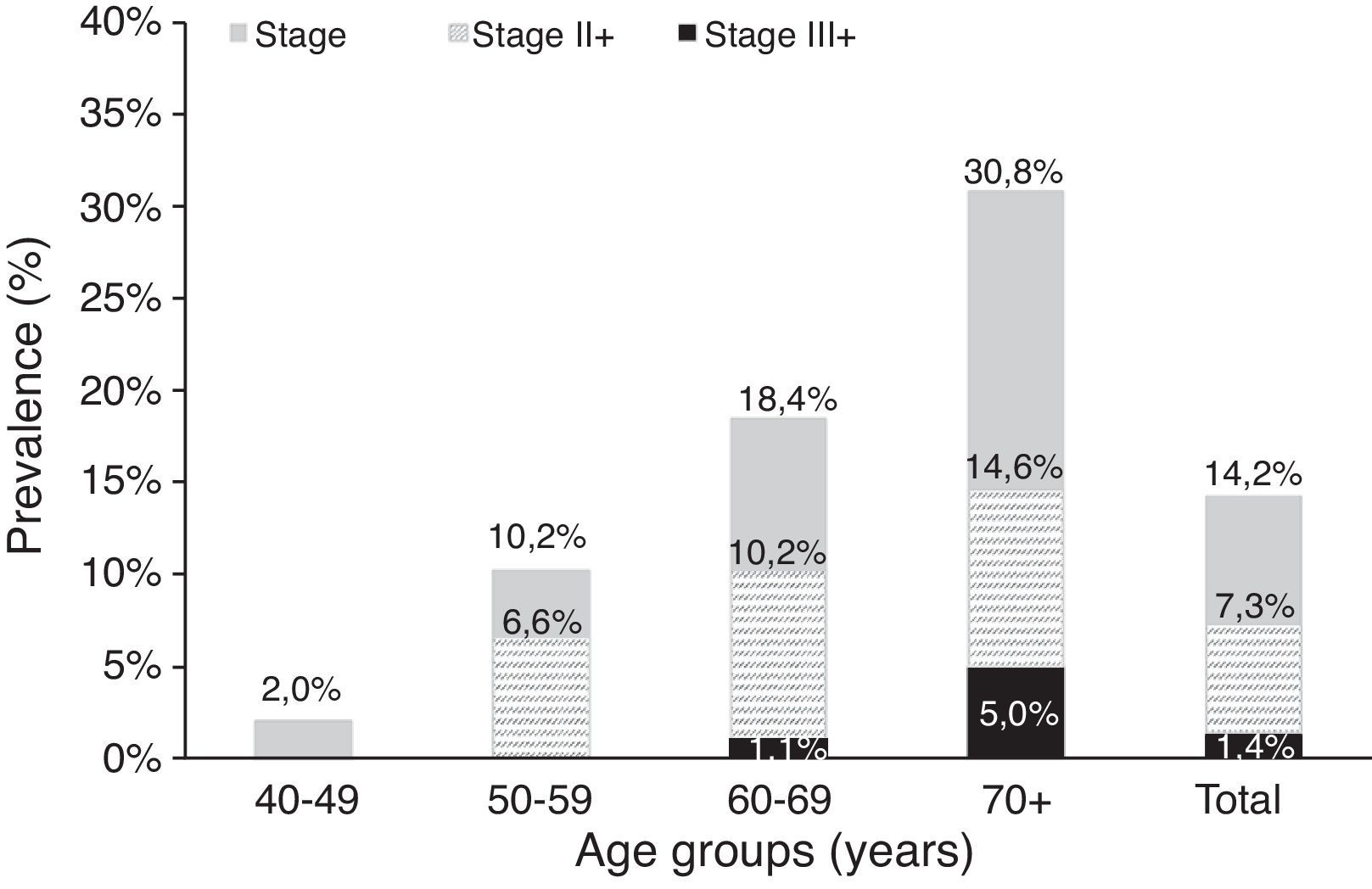
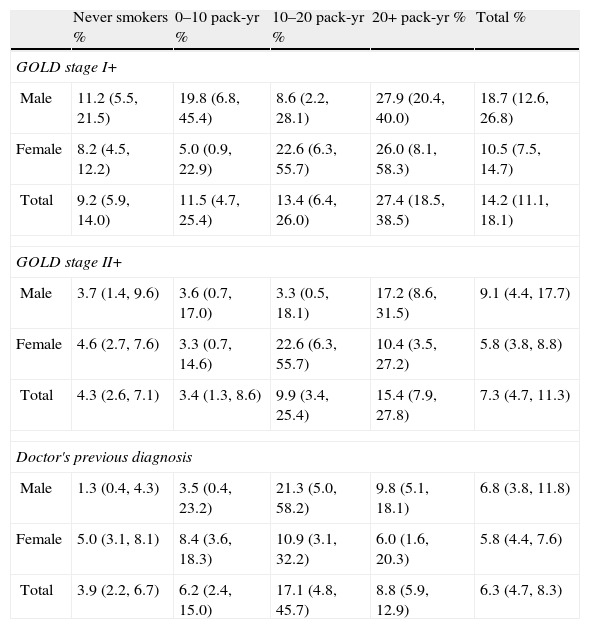
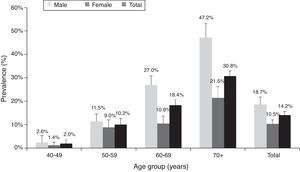
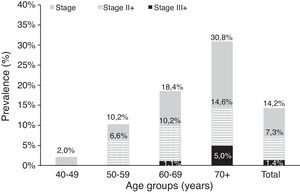
Table 4a (electronic appendix) shows the unweighted estimated prevalence of COPD by gender and pack years as 20.2% (95% C.I. 17.4, 23.3) and 9.5% (95% C.I. 7.6, 11.9) respectively for COPD stage I+ and II+. Overall, the weighted estimated-population prevalence for GOLD stage I+ COPD was 14.2% (95% C.I. 11.1, 18.1), 18.7% (95% C.I. 12.6, 26.8) for males and 10.5% (95% C.I. 7.5, 14.7) for females (Table 4). In individuals reporting respiratory symptoms the estimated population prevalence for GOLD stage I+ COPD was higher, respectively 19.9% (95% C.I. 8.7, 39.3) for chronic cough, 21.7% (95% C.I. 13.1, 33.7) for chronic phlegm, 23% (95% C.I. 17.3, 29.8) for wheezing and 22.7% (95% C.I. 11.5, 39.9) for dyspnoea (data not shown). Concerning GOLD stage II+ COPD the estimated prevalence was 7.3% (95% C.I. 4.7, 11.3), and 1.4% (95% C.I. 0.23, 2.47) for GOLD stage III+, with higher levels in older people (70+ years: 5%); it was absent in the age group 40–49 years (Figs. 2 and 3).
Estimated-population prevalence of COPD according to GOLD stage I+, GOLD stage II+ and doctor's previous diagnosis by gender and pack-years.*
| Never smokers % | 0–10 pack-yr % | 10–20 pack-yr % | 20+ pack-yr % | Total % | |
| GOLD stage I+ | |||||
| Male | 11.2 (5.5, 21.5) | 19.8 (6.8, 45.4) | 8.6 (2.2, 28.1) | 27.9 (20.4, 40.0) | 18.7 (12.6, 26.8) |
| Female | 8.2 (4.5, 12.2) | 5.0 (0.9, 22.9) | 22.6 (6.3, 55.7) | 26.0 (8.1, 58.3) | 10.5 (7.5, 14.7) |
| Total | 9.2 (5.9, 14.0) | 11.5 (4.7, 25.4) | 13.4 (6.4, 26.0) | 27.4 (18.5, 38.5) | 14.2 (11.1, 18.1) |
| GOLD stage II+ | |||||
| Male | 3.7 (1.4, 9.6) | 3.6 (0.7, 17.0) | 3.3 (0.5, 18.1) | 17.2 (8.6, 31.5) | 9.1 (4.4, 17.7) |
| Female | 4.6 (2.7, 7.6) | 3.3 (0.7, 14.6) | 22.6 (6.3, 55.7) | 10.4 (3.5, 27.2) | 5.8 (3.8, 8.8) |
| Total | 4.3 (2.6, 7.1) | 3.4 (1.3, 8.6) | 9.9 (3.4, 25.4) | 15.4 (7.9, 27.8) | 7.3 (4.7, 11.3) |
| Doctor's previous diagnosis | |||||
| Male | 1.3 (0.4, 4.3) | 3.5 (0.4, 23.2) | 21.3 (5.0, 58.2) | 9.8 (5.1, 18.1) | 6.8 (3.8, 11.8) |
| Female | 5.0 (3.1, 8.1) | 8.4 (3.6, 18.3) | 10.9 (3.1, 32.2) | 6.0 (1.6, 20.3) | 5.8 (4.4, 7.6) |
| Total | 3.9 (2.2, 6.7) | 6.2 (2.4, 15.0) | 17.1 (4.8, 45.7) | 8.8 (5.9, 12.9) | 6.3 (4.7, 8.3) |
According to Table 4, the population-estimated prevalence of GOLD stage I+ COPD was 9.2% (95% C.I. 5.9, 14.0) in participants who had never smoked and increased with the number of pack-years; it was 27.4% (95% C.I. 18.5, 38.5) in those with a smoking history of ≥20 pack-years. Similarly, the population-estimated prevalence of GOLD stage II+ COPD was 4.3% (95% C.I. 2.6, 7.1) in never smokers and 15.4% (95% C.I. 7.9, 27.8) in participants with ≥20 pack-year's smoking history.
Overall, the population-estimated prevalence of reported COPD doctor's diagnosis was much lower than COPD spirometrically defined (6.3% versus 14.2%). The prevalence of COPD doctor's dx was not associated with an increase of pack years of smoking (Table 4).
From the total group with COPD spirometric diagnosis, 86.8% did not report previous COPD doctor's dx (underdiagnosis). On the other hand, from the total group that reported previous COPD doctor's dx, 61.2% were not confirmed by spirometric analysis (overdiagnosis).
DiscussionThe main finding of our study is that COPD is a highly prevalent disease in Lisbon-Portugal, with an estimated prevalence of 14.2% in adults, aged 40 years or older. The overall prevalence of GOLD-defined COPD was higher in men than in women. COPD prevalence increased with age and smoking habits, with the highest estimated prevalence in men (47.2%) 70+ years old. Our data also support a high level of underdiagnosis (86.8%) and an unexpected high prevalence (9.2%) in never smokers.
It is necessary to quantify COPD prevalence, so as to document the effects of COPD effects on disability, quality of life and health care costs, and also to inform governments and public health authorities in planning to meet the growing demand for services.8,10 The BOLD initiative was developed standardized methods for estimating COPD prevalence and for obtaining data about risk factors. The first BOLD report of COPD prevalence across the world showed heterogeneity between countries and genders. Moreover, the reported prevalence tended to be greater than those previously known.8
The Lisbon BOLD survey is the first Portuguese study about COPD prevalence with a standardized methodology implemented internationally allowing comparison across countries. The main strength of this survey was the use of BOLD protocol, with a rigorous methodology to achieve the maximum accuracy and completeness of the survey and a high-quality post-BD spirometry. This methodology ensured that data were as easy to compare as possible with other BOLD studies.10
The reported low response rate (27%) of our study was one of the main limitations; this can be justified as a reactive attitude of participants to the high number of calls made by marketing companies in Portugal. Nevertheless, our response rate was similar to other published data sites (Vancouver and Kentucky),8 which also used random-digit-dialling.
In order to make sure that the studied sample was representative of the whole target population, a minimal data questionnaire was collected from the non responders, making it possible to compare both groups (responders/non responders). Except for gender, there were no differences between the groups in relation to age, risk factors and clinical profile. To deal with the high non-response rate and also gender differences, more telephone contacts were made to obtain data from at least 300 females and 300 males. In addition, in order to overcome the potential for response bias, we used weighting for the adjustment of prevalence to the target population.10
Before the BOLD results became available, Portuguese official prevalence of COPD was 5.34%, based on a study from 2002.6 Portugal Lisbon BOLD data revealed a higher value of COPD prevalence (14.2%). So far, all the projections and health planning regarding COPD have been based on the previous prevalence figures meaning that the real burden of COPD has been underestimated.
Because the two studies used different methodologies, their results should not be compared.14 Regarding the 2002 study, there were some methodological issues that make comparison impracticable. In fact, this study used a different source population, a restrictive sample in relation to age range and also different COPD diagnosis criteria (without post-BD spirometry) and reference equations.
Comparing our data to the international BOLD studies we conclude that Portuguese COPD prevalence is lower than many countries prevalence,2,8,16–19 although similar to the prevalence of some European countries like Germany (13.2%)20 and Sweden (16.2%).21 These differences could be attributed to different levels of smoking in the population, or possibly other risk factors, which are not analyzed here and might need further investigation (e.g. occupation, biomass, air pollution).
In our study, NHANES III equations were used to estimate the prevalence of COPD Stage II+, in order to allow comparisons between countries. However it should be noted that the choice of the right setting of predicted equations is a matter of debate and might influence prevalence estimates.
Our data showed that COPD prevalence increases with age, being higher above 70 years old. It is worth noting that, with ageing, the prevalence in men becomes twice the prevalence in women of the same age group, probably reflecting ever smoking prevalence that corresponds to the cumulative effect of tobacco. Taking into account that COPD stage I+ is defined by the fixed ratio FEV1/FVC, and that this ratio falls with age in healthy individuals,22 the high prevalence found in older people could also represent overdiagnosis (47.2% in stage I+ versus 17.2% in stage II+). Nevertheless, we still found the same tendency for age related increase in COPD prevalence in stage II+, although less pronounced.
In the group of females with 10–20 pack-yr the prevalence is exactly the same for GOLD stage COPD I+ and II+ (Table 4), meaning that, in our sample, there were no women in the group with a FEV1/FVC<70% and FEV1>80% predicted. Although unusual, this data was double-checked and confirmed.
As found in other studies,8,15,18–20 there was also a positive trend with the increasing of pack-years, confirming smoking as an important risk factor for the development of the disease.12 In fact, above 20 pack-years COPD prevalence doubles (27.4% versus 14.2%), representing an even higher burden of healthcare resources utilization and costs.23
Another important issue of this study was the estimate of 17.9% for the prevalence of current smoking in Portuguese people older than 40 years old. This data is consistent with the official prevalence data (17.2%) from the National Health survey of 2005/2006.24 The highest prevalence of smoking habits found in younger women (40–49 years) strengthens the future projection of an increase of COPD in women, and also the need to target teenagers and young women in smoking cessation campaigns. Concerning never smokers, an unexpected COPD prevalence of 9.2% was found, suggesting a possible overdiagnosis by spirometric parameters. Nevertheless, the COPD prevalence of 4.3% for stage II+, could be the expression of other risk factors that should also be investigated (e.g. biomass exposure, childhood respiratory tract infections, past tuberculosis). Moreover in some people, this high prevalence might indicate asthma with remodelling in the small airways.8 Similar findings have been described in other countries.5
The finding of higher levels of COPD prevalence in symptomatic subjects than in the general population was also expected12 and the need for a spirometry should be brought to the attention of primary care physicians.
In this study, a gap was found between the presence of airflow obstruction defined by GOLD criteria and doctor's diagnosis. Overall, only 6.3% of the participants reported COPD doctor's diagnosis, while GOLD stage I+ registered 14.2%. Moreover, the difference is even greater if we base our analysis on the degree of concordance between COPD doctor diagnosis and spirometric diagnosis. In fact, only 13.2% of spirometrically diagnosed COPD had been previously diagnosed (underdiagnosis). These data are consistent with those of Spain where, despite an eventual decrease in COPD prevalence, there are still high levels of underdiagnosis.25–27 Furthermore, 61.2% of the reported cases of “COPD” declared by the participants to have been “doctor diagnosed” were not confirmed by spirometry (overdiagnosis). These numbers clearly show a high degree of COPD misdiagnosis and highlight the urgency to improve physicians’ knowledge about COPD diagnosis, and the need to emphasize the use of spirometry, particularly with symptomatic subjects.
ConclusionsThe 14.2% estimated-prevalence indicates that COPD is a common disease in the Lisbon region. The high prevalence of COPD with a large proportion of undiagnosed disease, highlights the importance of raising awareness of COPD among health professionals, and requires more use of spirometry in the primary care setting. Despite ageing and smoking remain major risk factors for COPD, other risk factors contributing to the presence of disease in never smokers should be investigated in future studies.
FundingThe BOLD project in Portugal was conducted by the Respiratory Portuguese Society to the Co-ordinating Centre located at the Imperial College, UK and funded by Boehringer Ingelheim and Pfizer.
Conflicts of interestThe BOLD project in Portugal was conducted by the Respiratory Portuguese Society to the Co-ordinating Centre located at the Imperial College, UK and funded by Boehringer Ingelheim and Pfizer.
The BOLD investigators would like to thank:
- -
Sonia Buist (Oregon Health and Sciences University, Portland, OR, USA), William Vollmer, Mary Ann McBurnie and Suzanne Gillespie (Kaiser Permanente Center for Health Research, Portland, OR, USA) and Robert Jensen (Latter Day Saints Hospital, Salt Lake City, UT, USA) for all the support to Fátima Rodrigues and Hermínia Dias in BOLD protocol, including questionnaires and spirometry certification and questionnaires validation for Portuguese language.
- -
The UK Co-ordinating Centre staff particularly Richard Hooper, Anamika Jithoo; and Sonia Coton.
- -
The certified interviewers: Ana Bandarra, Andreia Machado, João Silva, Tânia Fonseca and Ângela Antunes.
- -
The certified physicians: Inês Faria, Ana Sofia Oliveira, Márcia Man, Patrícia Garrido, Ana Margarida Alves and Susana Carreira, who participated with Fátima Rodrigues in the revision of questionnaires and submission of data to BOLD UK Centre.
- -
The certified cardiopulmonology technicians: Ana Cristina Lutas, Ana Sofia Cachola, Andreia Carina de Jesus Colaço, Andreia Descalço, Cátia Lígia Oliveira, Cláudia Pereira, Daniela Ricardo, Joana Quaresma, Marta Landeiro, Sara Matos, Sara Serranho and Susana Filipa Gomes Marcelino, from Escola Superior de Tecnologia da Saúde de Lisboa/Instituto Politécnico de Lisboa.
BOLD investigators would also like to thank Prof. Doutor António Gouveia for the statistical support and Carlos Capela, as sponsor project leader, and everybody at Boehringer Ingelheim Portugal and Pfizer who were involved and collaborated in this project.
Please cite this article as: Prevalência da doenc¸a pulmonar obstrutiva crónica em Lisboa,Portugal: estudo Burden of Obstructive Lung Disease. 2013. http://dx.doi.org/10.1016/j.rppneu.2012.11.004.