Gorham-Stout disease is an extremely rare disease of unknown etiology. It is characterized by the local proliferation and dilation of lymphatic vessels, which causes spontaneous progressive osteolysis with bone resorption. It may affect one or several – usually contiguous – bones, generally the hips, the scapular waist, the vertebrae, the ribs and the skull. It mainly affects children and young adults, with no gender-based differences. Diagnosis is based on clinical, analytical, radiological and histopathological findings. Chylothorax may occur as a result of the involvement of the lymphatic vessels of the pleura or thoracic duct by adjacent osteolysis.1
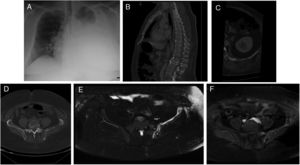
A 37 year-old man presented with a 14-day history of progressive dyspnea that occurred even with minimal effort. The patient had normal vital signs, hypoxemia, and a generalized reduction of respiratory sounds in the left hemithorax. Blood analysis was normal. Chest X-ray showed a massive left pleural effusion without displacement of the midline (Fig. 1A). The radiological findings of CT scan and MRI are shown in Fig 1B–F.
(A) Chest X-ray PA showed a radiodensity practically diffuse of the left hemitorax in relation to severe pleural effusion. (B) The sagital reformatted CT image demonstrated a diffuse fat infiltration with loss of the trabecular bone pattern in the vertebral bodies T12 and L4 and an infiltration of the deep subcutaneous cellular tissue in the posterior abdominal wall. (C) The axial reformatted CT image showed multiple fatty lytic bone lesions with cortical disruptions in the 12th left rib. (D) Axial CT imagen demonstrated an incomplete osteolysis of the left iliac bone with multiple fatty lytic bone lesions with cortical disruptions and without an increase of soft tissues. (E) Axial TSE-T2 weighted fat-supressed image showed a diffuse hyperintensity of the left iliac bone, where linear structures are recognized that correspond to the dilatation of the lymphatic vessels. (F) This lesion presented an intense diffuse enhancement after administration of IV contrast on an axial TSE-T1 weighted fat-supressed image.
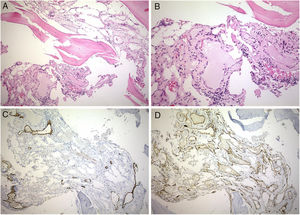
The milky pleural fluid was a lymphocyte-predominant exudate (93% of lymphocytes); its biochemistry was compatible with chylothorax (1452mg/dL of triglycerides). Malignancy was excluded after several negative pleural biopsies. Talc pleurodesis was performed. Microscopic findings on iliac bone biopsy are shown in Fig. 2. Sirolimus therapy was started at a dose of 2.5mg taken orally twice a day, which was titrated to 2mg orally twice a day based on a trough level goal of 9–12mcg/L, with no side effects. Since then and nine months after the onset of symptoms, under analytical and radiological monitoring, the patient remains asymptomatic, without changes in complementary tests.
Bone biopsy. Gorham disease composed of numerous vascular spaces lined by attenuated endothelium. (A) Hematoxylin–eosin (10×). (B) Hematoxylin–eosin (20×). (C) Immunohistochemistry stain for D2-40 in endothelial cell of lymphatic vessels (10×). (D) Immunohistochemistry stain for CD31 in endothelial cells of capillary vessels (10×).
The sequence of bone loss secondary to Gorham-Stout disease is: early intraosseous stage with irregular (or patched) osteoporosis and subsequent appearance of intramedullary and subcortical radiolucency; a late extraousseous stage where the bone cortex breaks resulting in bone destruction, resorption and loss. The induced osteolysis is accompanied by vascular malformations (capillary, venous and lymphatic alterations). Alterations are not neoplastic but developmental, they aggravate with time and can be found in numerous tissues.2 Pathologically, lesions are characterized by the lack of proliferation of endothelial cells3; histologically, by a marked proliferation and dilation of lymphatic vessels, and clinically by the late onset and lack of spontaneous remission.2
CD31 and D2-40 are markers of lymphatic endothelial competence during development.4 Therefore, CD31 and D2-40-positive endothelial cells suggest that the proliferating vasculature primarily stems from the lymphatic endothelium. There may be an increase in circulating levels of platelet-derived growth factor receptor-beta, which means that the growth factor may also have a relevant role in this entity.
The prevalence of pleural effusion in these patients ranges from 17 to 42%.1,5 Yet, as in this case, chylothorax is not generally the first sign of the disease. The onset generally presents as bone pain or spontaneous fractures. There have been no studies large enough published to report the characteristics of chylothorax associated with Gorham-Stout disease. In our case, chylothorax was characterized by a lymphocyte-predominant exudate without other significant findings. Chylothorax is generally bilateral, although it can affect any side. It affects men and women equally.
As Gorham-Stout disease is a very rare disease and its underlying pathogenic mechanisms are unknown, there is no standardized treatment for the disease, and prognosis is unclear. Although some patients remain stable, those with chylothorax seem to have a poorer prognosis. In one review, 17 of the 39 patients with chylothorax, (43.6%) died.5 There are few publications assessing the clinical course of the disease with different treatments. The disease has been reported to have stabilized spontaneously in some patients. Although anecdotal, treatments with bisphosphonate (based on their anti-osteoclastic properties), alfa-2b interferon6 and sirolimus have been documented.
Surgical procedures for chylothorax include pleurectomy, pleurodesis7 and ligation of the thoracic duct.1 In a series of 25 patients, 11 underwent thoracic duct ligation. Disease evolution was good in seven patients, who survived (63.6%). Surgery failed in four patients whose thoracic duct could not be found. Nine of the 14 patients who did not undergo surgery or pleurodesis died (69%). Two of the three patients who underwent pleurodesis survived. The other patient died.1 Surgery should be performed in initial stages of the disease to avoid malnutrition and immunosuppression secondary to lymphopenia. However, chylothorax cannot be always resolved in the long term. Radiotherapy can be administered for chylothorax to patients who cannot undergo surgery due to their poor general state or surgery failure. Side effects of radiotherapy include post-radiation pneumonitis, gastrointestinal disorders, growth alterations and appearance of a secondary neoplasm.
To sum up, Gorham-Stout disease is a rare musculoskeletal disease that should be considered in the presence of chylothorax even as a first sign. Prognosis is unknown and no effective therapies have yet been developed. Definitive diagnosis should be based on a biopsy of the lesion after other causes of osteolysis have been excluded.
Author's contributionsLF, JSA and LV were responsible for the conception and design of the study, and wrote and edited the manuscript. CR, IAN, ABN and AFG contributed to the drafting and revision of the manuscript. All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.