Asthma may result in postural disorders due to increased activity of accessory respiratory muscles and hyperinflation. Our primary objective was to assess the correlation between pulmonary function and posture in adult patients with asthma. Secondarily, we aimed to study the correlation between body composition and body posture in this group of patients.
MethodThis was a cross-sectional study including 34 patients with asthma who were subjected to postural assessment (photogrammetry), pulmonary function testing (spirometry, whole-body plethysmography, diffusing capacity for carbon monoxide, and respiratory muscle strength), and body composition estimation by means of bioelectrical impedance.
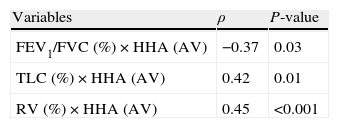
ResultsMost patients were female (70.6%) with a median age of 32.5 years (range: 23–42 years old). We found a significant correlation between horizontal alignment of head (anterior view) and the ratio of forced expiratory volume in 1s to forced vital capacity (FEV1/FVC; ρ=−0.37; P=0.03), total lung capacity (TLC; ρ=0.42; P=0.01), and residual volume (RV; ρ=0.45; P<0.001). Bronchial obstruction and respiratory muscle strength variables also correlated with postural assessment measures on the right and left lateral views. Both body mass index and the percentage of fat mass were correlated with horizontal alignment of head, horizontal alignment of the pelvis, and the frontal angle of the lower limbs.
ConclusionAdult patients with asthma exhibit specific postural disorders that correlate with pulmonary function and body composition. The assessment of postural variables may provide a better pulmonary rehabilitation approach for these patients.
A asma pode resultar em alterações posturais causadas pelo aumento da atividade da musculatura acessória, respiratória e insuflação pulmonar. Nosso objetivo primário foi avaliar a correlação entre função pulmonar e postura em pacientes adultos com asma. Como segundo objetivo buscou-se estudar a correlação entre composição corporal e postura neste grupo de pacientes.
MétodosFoi realizado um estudo transversal onde 34 pacientes com asma se submeteram à avaliação da análise postural (fotogrametria), função pulmonar (espirometria, pletismografia de corpo inteiro, medição da capacidade de difusão do CO e força muscular respiratória) e composição corporal através da bioimpedância elétrica.
ResultadosA maioria dos pacientes era do sexo feminino (70,6%), com mediana da idade de 32,5 anos (variação: 23-42 anos). O alinhamento horizontal da cabeça (vista anterior) correlacionou de forma significante com as seguintes variáveis: relação entre o volume expiratório máximo no primeiro segundo e a capacidade vital forçada (VEMS/CVF) (ρ = −0,37; P = 0,03); capacidade pulmonar total (CPT) (ρ = 0,42; P = 0,01); e volume residual (VR) (ρ = 0,45; P < 0,001). Os indicadores de obstrução brônquica e força muscular respiratória correlacionaram-se também com as medidas de avaliação postural obtidas em vista lateral direita e esquerda. Tanto o índice de massa corporal quanto o percentual de massa gorda correlacionaram-se com alinhamento horizontal da cabeça, alinhamento horizontal da pélvis e ângulo frontal do membro inferior.
ConclusõesPacientes asmáticos adultos apresentam alterações posturais específicas que correlacionam com a função pulmonar e com a composição corporal. A avaliação das medidas posturais pode fornecer uma melhor abordagem para a reabilitação pulmonar nestes pacientes.
Asthma is a chronic respiratory disease characterized by increased bronchial responsiveness, reversible airflow obstruction, and bronchial inflammation, which is considered the main physiopathogenic aspect of the disease.1,2 The World Health Organization estimates that 235 million people suffer from asthma worldwide.3
Posture is defined as a balanced arrangement of the body structures determined by the position of all the body segments at a given moment.4 In normal postural alignment, the muscles and joints are expected to be in a state of equilibrium while exhibiting minimal effort and overload.5 The postural stance associated with thorax hyperinflation might induce a series of compensations involving the vertebral column and the scapular belt and pelvic girdle. Thus, patients with chronic respiratory diseases may develop changes in posture and balance.6–9
Patients with asthma exhibit excessive respiratory muscle recruitment in response to airflow obstruction, which results in adaptive hypertrophy.6,7 Because these muscles are repeatedly under tension, they become shorter and lose flexibility.10 However, since there is a biomechanical interdependence in the human locomotor system, any mechanical abnormality in the thoracic cage influences the overall body mechanics.11 In this sense, postural assessment is of fundamental importance for the diagnosis, planning, and monitoring of the progress and results of physical therapy.
Obesity is a common condition among adults with asthma and is usually associated with symptom exacerbation and use of oral corticosteroids.12,13 Obese asthmatic individuals exhibit reduced pulmonary function and poorer quality of life than eutrophic patients.14 Population-based studies suggest that high values of body fat and low values of fat-free mass (FFM) are predictors of mortality that are independent of the underlying disease.15 In addition, the percentages of fat mass (FM) and FFM directly correlate with pulmonary function. It has been demonstrated that weight loss and body mass index (BMI) reduction is associated with improvement in pulmonary function.16
Asthma might cause systemic repercussions due to its severity and the effects of treatment.12,13 Our hypothesis is that, in adults with asthma, the changes in respiratory mechanics and obesity interfere with body posture. We believe that knowledge about the impact of pulmonary function abnormalities and obesity on body posture can help prevent postural impairments in these patients. Thus, our primary objective was to assess the correlation between pulmonary function and posture. Secondarily, we aimed to investigate the correlation between body composition and body posture in this group of patients.
MethodsPatientsWe conducted a cross-sectional study with adult patients recruited at the Newton Bethlem Community Health Clinic in the city of Rio de Janeiro, Brazil. Patients aged 18–50 years old who were diagnosed with asthma were included. Patients who were ex-smokers, used psychotropic drugs or had a history of respiratory comorbidities were excluded. The present study was approved (n° 012/2011) by the Ethics Committee of the institution, and all participants signed informed consent forms.
MeasurementsPostural assessment was performed by means of photogrammetry using the postural assessment software (PAS) (FAPESP Virtual Incubator, SP, Brazil).17 Coordinates of anatomical points (right and left tragus, right and left acromion, right and left greater trochanter of the femur, and spinous processes of the seventh cervical and third thoracic vertebrae) were labeled with passive markers (styrofoam balls attached with double-sided adhesive tape), and photographs of the anterior, posterior, and right and left lateral views were taken for all participants. Then, the photographs were transferred to a compatible microcomputer and analyzed.
The following tests were performed using the computerized Collins Plus Pulmonary Function Testing Systems (Warren E. Collins, Inc., Braintree, MA, USA): spirometry, whole-body plethysmography, measurement of diffusing capacity for carbon monoxide (DLco), and assessment of respiratory muscle strength. All spirometric tests followed the standards and interpretation of the American Thoracic Society (ATS).18 Pereira's (spirometry) and Neder's (lung volumes, DLCOsb, and muscle strength) equations were used for interpreting functional parameters.19–22
Body composition was analyzed using a bioelectrical impedance device (BIA 310e, Biodynamics, Seattle, WA, USA). The participants were instructed to rest for 5min before the exam. During the test they were barefoot, away from metallic objects, and stood with their feet 15–30cm apart.23 Two electrodes were placed on the dorsum of the right hand, and two were placed on the dorsum of the right foot. Resistance and reactance were calculated and used to estimate FFM. The selected equation has been previously validated for Brazilian individuals: FFM=−4.104+(0.518×height2/resistance)+(0.231×weight)+(0.130×reactance)+(4.229×gender: male=1, female=0).23
Data analysisData are given as median and interquartile ranges (25% and 75% percentiles) or as frequencies (percentages). Because the Shapiro–Wilk test determined that the variables were not distributed normally, correlations were assessed with Spearman's test. Analysis was performed using SigmaStat for Windows, version 3.5 (Systat Software, Inc., Chicago, IL, USA). Statistical significance was established as P<0.05.
ResultsOf the forty-six patients with asthma who were initially screened, 12 were excluded for the following reasons: declining to participate (3), associated restrictive disease (4), inability to perform the pulmonary function testing (4), and death (1).
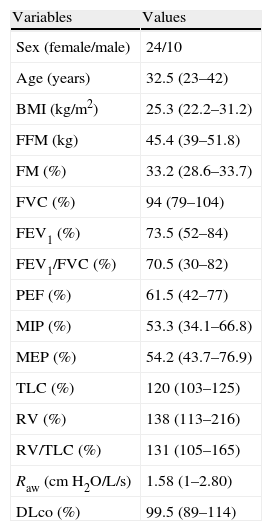
The anthropometric, body composition, and pulmonary function data are described in Table 1, and postural assessment data are shown in Table 2. Although there was a slight discrepancy between the mean values of the right and left sides, the differences investigated with PAS were not statistically significant (P>0.05).
Anthropometry, body composition, and data on the pulmonary function of the investigated sample (n=34).
| Variables | Values |
| Sex (female/male) | 24/10 |
| Age (years) | 32.5 (23–42) |
| BMI (kg/m2) | 25.3 (22.2–31.2) |
| FFM (kg) | 45.4 (39–51.8) |
| FM (%) | 33.2 (28.6–33.7) |
| FVC (%) | 94 (79–104) |
| FEV1 (%) | 73.5 (52–84) |
| FEV1/FVC (%) | 70.5 (30–82) |
| PEF (%) | 61.5 (42–77) |
| MIP (%) | 53.3 (34.1–66.8) |
| MEP (%) | 54.2 (43.7–76.9) |
| TLC (%) | 120 (103–125) |
| RV (%) | 138 (113–216) |
| RV/TLC (%) | 131 (105–165) |
| Raw (cmH2O/L/s) | 1.58 (1–2.80) |
| DLco (%) | 99.5 (89–114) |
Results expressed as median (interquartile range) or number (%).
BMI: body mass index; FFM: fat-free mass; FM: fat mass; FVC: forced vital capacity; FEV1: forced expiratory volume in 1s; MIP: maximal inspiratory pressure; MEP: maximal expiratory pressure; PEF: peak expiratory flow; TLC: total lung capacity; RV: residual volume; Raw: airway resistance; DLco: diffusing capacity for carbon monoxide.
Values of postural assessment software of patients with asthma (n=34).
| Variables | Values |
| HHA (AV) | 1.65 (2.20–3.70) |
| Right | Left | |
| HHA | 39.3 (35.8–45) | 39.5 (36.5–45) |
| LLFA | 4.90 (1.40–7.30) | 4.40 (0.50–7) |
| HA | 18.3 (9.40–24.8) | 23.4 (14.5–33.6) |
| TVA | 4.80 (3.50–7.70) | 4 (2.50–8.30) |
| PHA | 13.7 (8.70–19.7) | 13.8 (10.5–19.9) |
| KA | 4.90 (4.50–5.40) | 4.90 (1.70–8.10) |
| AA | 83.8 (81.2–86.4) | 87.4 (82.9–90) |
Results expressed as median (interquartile range) or number (%).
HHA (AV), head – horizontal alignment (anterior view); LLFA, lower limbs frontal angle; HA, hip angle; TVA, trunk – vertical alignment; PHA, pelvis – horizontal alignment; KA, knee angle; AA, ankle angle.
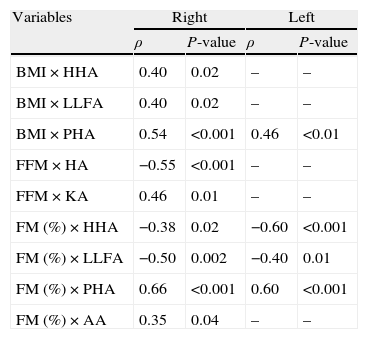
Table 3 describes the correlation between body composition and postural assessment, whereas Table 4 shows the correlation between pulmonary function and postural assessment; only the statistically significant data are shown in both tables. A significant correlation was found between postural deviations and indicators of bronchial obstruction, including reduction of the ratio of forced expiratory volume in 1s to forced vital capacity (FEV1/FVC), reduction of the peak expiratory flow (PEF), increased total lung capacity (TLC) and residual volume (RV), and increased airway resistance (Raw). Postural assessment parameters also exhibited significant correlation with respiratory muscle strength measurements.
Correlation between body composition and postural assessment measurements (Spearman's correlation test, showing only statistically significant correlations) (n=34).
| Variables | Right | Left | ||
| ρ | P-value | ρ | P-value | |
| BMI×HHA | 0.40 | 0.02 | – | – |
| BMI×LLFA | 0.40 | 0.02 | – | – |
| BMI×PHA | 0.54 | <0.001 | 0.46 | <0.01 |
| FFM×HA | −0.55 | <0.001 | – | – |
| FFM×KA | 0.46 | 0.01 | – | – |
| FM (%)×HHA | −0.38 | 0.02 | −0.60 | <0.001 |
| FM (%)×LLFA | −0.50 | 0.002 | −0.40 | 0.01 |
| FM (%)×PHA | 0.66 | <0.001 | 0.60 | <0.001 |
| FM (%)×AA | 0.35 | 0.04 | – | – |
BMI: body mass index; HHA: head – horizontal alignment; LLFA: lower limbs frontal angle; PHA: pelvis – horizontal alignment; FFM: fat-free mass; HA: hip angle; KA: knee angle; FM: fat mass; AA: ankle angle.
Correlation between pulmonary function values and postural assessment measurements (Spearman's correlation test, showing only statistically significant correlations) (n=34).
| Variables | ρ | P-value |
| FEV1/FVC (%)×HHA (AV) | −0.37 | 0.03 |
| TLC (%)×HHA (AV) | 0.42 | 0.01 |
| RV (%)×HHA (AV) | 0.45 | <0.001 |
| Right | Left | |||
| FEV1 (%)×TVA | 0.35 | 0.04 | – | – |
| FEV1 (%)×KA | – | – | −0.38 | 0.03 |
| FEV1/FVC (%)×KA | – | – | −0.38 | 0.02 |
| PEF (%)×PHA | −0.48 | <0.01 | −0.41 | 0.02 |
| PEF (%)×KA | – | – | −0.50 | <0.01 |
| PEF (%)×AA | – | – | −0.37 | 0.03 |
| MIP (%)×TVA | −0.46 | <0.01 | −0.40 | 0.02 |
| MIP (%)×HA | −0.51 | <0.001 | −0.43 | 0.01 |
| MIP (%)×PHA | −0.43 | 0.01 | −0.45 | <0.01 |
| MEP (%)×PHA | −0.43 | 0.01 | −0.37 | 0.03 |
| MEP (%)×TVA | −0.47 | <0.01 | – | – |
| MEP (%)×HA | −0.43 | 0.01 | – | – |
| TLC (%)×TVA | 0.45 | <0.01 | – | – |
| RV (%)×TVA | 0.49 | <0.01 | – | – |
| RV (%)×HA | – | – | 0.37 | 0.03 |
| Raw (cmH2O/L/s)×HA | 0.46 | <0.01 | – | – |
FEV1: forced expiratory volume in 1s; FVC: forced vital capacity; HHA (AV): head – horizontal alignment (anterior view); TLC: total lung capacity; RV: residual volume; TVA: trunk – vertical alignment; KA: knee angle; PHA: pelvis – horizontal alignment; PEF: peak expiratory flow; AA: ankle angle; MIP: maximal inspiratory pressure; HA: hip angle; MEP: maximal expiratory pressure; Raw: airway resistance.
The main findings of the present investigation were that, in adult patients with asthma, there is a close association between postural deviations and airways obstruction variables, including ‘air trapping’ and hyperinflation. Also, both respiratory muscle strength and body composition contribute to the abnormalities in body posture. To the best of our knowledge, this study is the first to assess the relationship between pulmonary function, posture, and body composition in this group of patients.
Posture assessment in a standing position has been increasingly used in clinical practice, as the body alignment observation can be used to plan and monitor physical therapy treatments. Photogrammetry by means of the PAS has been used to assess the correlations between posture, pulmonary function, and body composition.24–26
Compared to normal values,5 we found forward head posture and increased lumbar lordosis among asthmatic individuals, as well as bilateral hip and knee flexion. Although a slight discrepancy was found between the right- and left-side values, the variables investigated by PAS did not exhibit significant differences (P>0.05). This relative symmetry can be justified because asthma affects the lungs diffusely and patients with comorbidities which might cause lateral posture deviations were excluded from the present study.
With regard to body composition, some postural changes such as the horizontal alignment of head and lower limb frontal angle correlated positively with BMI and negatively with FM (%), whereas the angle of the knee exhibited a significant positive correlation with FFM. The associations between body composition and postural deviations in the lower half of the body were probably because of the greater distribution of body fat in the hips. This distribution pattern of body fat (gynoid pattern obesity) is a common finding among females, who represent the majority of our sample because of the higher prevalence of asthma among female adults.27,28 Interestingly, Beckett et al.27 showed that the asthma is associated with weight gain in women but not in men, independent of physical activity; thus, we think that this finding may have influenced our results.
Robles-Ribeiro et al.29 observed that adult patients with mild to severe asthma exhibit several postural changes. They also noted that shoulder protraction in these patients increased as PEF decreased. However, the present study did not find a correlation between these postural changes and pulmonary function.
When assessing the correlation between postural variables and respiratory maximum pressures, we found a prevalence of chest and pelvic changes (trunk vertical alignment – right/trunk vertical alignment – left; horizontal alignment of the pelvis – right/horizontal alignment of the pelvis – left; right hip angle/left hip angle), which were negatively correlated with MIP and MEP. Abdominal weakness is known to exacerbate lumbar lordosis in children, mainly due to alterations of the oblique and transverse abdominal muscles.30 This group of muscles plays an important role in vertebral stabilization and forced expiration; therefore, it interferes with MEP measurements. In the present study we observed the same phenomenon in adults.
Boulay et al.31 observed that the postural behavior of the hyperinflated thorax induces a series of compensations in the thoracic spine, pelvic girdle, and scapular belt because the diaphragm becomes rectified and shortened. Because the diaphragm is directly connected to the endothoracic fascia, thoracic hyperinflation might results in the increase of thoracic kyphosis. We cannot claim that thoracic kyphosis was increased in our sample because the postural assessment software does not analyze this variable. However, we can confirm that, due to the connection of the diaphragm to other thoracic muscles such as the iliopsoas, transverse abdominals, and quadratus lumborum, asthma may lead to pelvic anteversion and lumbar hyperlordosis, which was observed in our sample of patients with asthma and also correlated with lung volumes.11 When evaluating the static lung volumes and Raw in patients with asthma, the most striking phenomenon is the ‘air trapping’ that is the consequence of a delayed alveolar emptying. The TLC can also be increased due to the loss of elastic recoil. Another mechanism involved in TLC increase is the shortening of the expiratory time in patients with asthma, so that inspiration begins before air is entirely expelled from the lungs. These findings might explain the correlations of TLC and Raw with the modifications of vertical alignment of the trunk observed in the present study.
Unlike Boulay et al.31, we did not find a correlation between pelvic misalignment and lung volume. However, we did observe a negative correlation of pelvic misalignment with muscle strength and peak flow, and we observed higher values on the right side for all variables. Our hypothesis is that there is a difference in the extent of pulmonary parenchyma damage. Greater alveolar destruction in one lung results in increased ‘air trapping’ and consequent rectification and shortening of the ipsilateral diaphragm, intercostal, and abdominal muscles. These one-sided changes might favor muscle imbalance and result in pelvic unleveling.
Increased DLco has been described in patients with asthma, including those with stable disease.32,33 Several mechanisms have been proposed as possible explanations for this abnormality, including increased perfusion of lung apices due to increased pulmonary arterial pressure or more negative pleural pressure as a consequence of bronchial narrowing.32 In the present study, we did not find any correlations among DLco, body composition, or postural disorders.
A critical analysis of the results and their limitations is appropriate. Respiratory and peripheral muscle strength and body composition are affected by oral steroid use, which is commonly prescribed to patients with asthma. Therefore, such medications might be a confounding factor. Due to the lack of a control group, we could not establish the influence of corticosteroids in the investigated sample. In addition, the present study is a cross-sectional analysis. It only indicates associations, it does not establish of cause-effect relationships. However, because there are few published studies regarding body posture of this group of patients, we believe that our results are an important contribution to the field.
In conclusion, our study demonstrates that adult patients with asthma exhibit specific postural disorders such as thoracic hyperkyphosis and lumbar hyperlordosis. These postural abnormalities correlate with patients’ pulmonary function and body composition. The assessment of postural variables may provide a better pulmonary rehabilitation approach for these patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Authorship/collaboratorsVívian Pinto de Almeida, Fernando Silva Guimarães, Sara Lucia Silveira de Menezes, Thiago Thomaz Mafort and Agnaldo José Lopes contributed to the conception and project, article review and approval of the final version of the manuscript. Vanessa Joaquim Ribeiro Moço carried out the analysis and interpretation of data, article review and approval of the final version of the manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Almeida VP, Guimarãesa FS, Moçoa1 VJR, Menezesa SLS, Mafortb TT, Lopes AJ. Correlação entre função pulmonar, postura e composição corporal em pacientes com asma. Rev Port Pneumol. 2013;19:204–210.
Role in the study: Analysis and interpretation of data, revising the article, and final approval of the version of manuscript.