Osteoprotegerin (OPG) is a member of the tumor necrosis factor family and a key regulator in bone turnover; it plays a role in the development of many cardiovascular diseases and may be treated as a marker of vascular damage. Bioelectrical impedance analysis (BIA) is a reliable, non-invasive and effective technique for measuring body composition.
The aim of the study was to evaluate correlations between osteoprotegerin serum levels and body composition parameters in sleep apnea patients and their influence on cardiovascular risk.
Material and methodsA total of 125 patients with newly diagnosed OSA were enrolled in the study (including 34 females). The mean age was 54.48±8.81 years, mean AHI 33.16±20.44/h and mean BMI 33.76±7.18. A control group comprised 59 healthy subjects with mean age of 51.27±12.97 years and mean BMI 29.47±5.42.
All subjects underwent a nocturnal respiratory polygraphy and body composition measurements were taken with bioelectrical impedance analysis. OPG serum levels were measured using the enzyme-linked immunosorbent assay (ELISA) method.
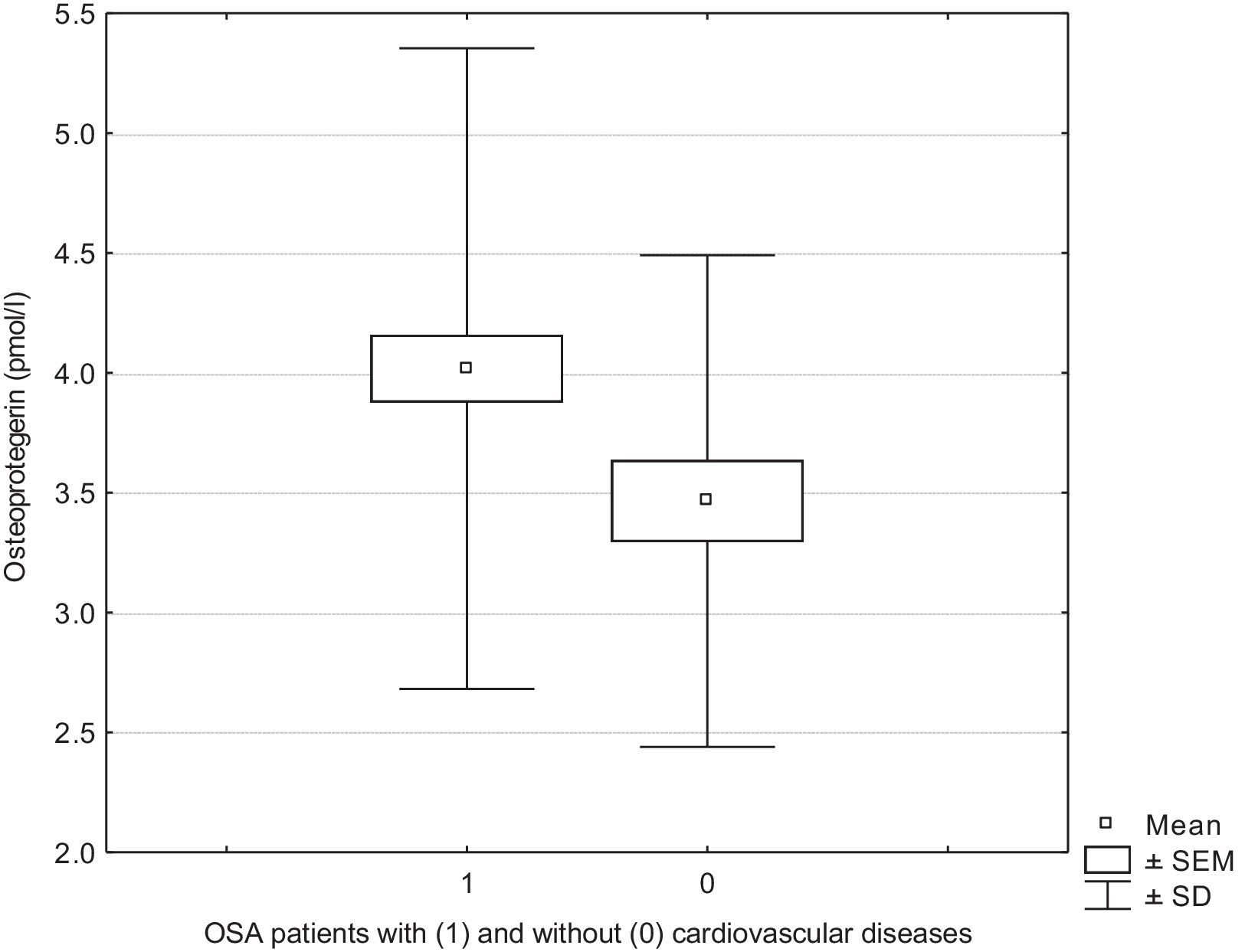
ResultsIn OSA patients OPG correlated negatively with muscle mass percentage (MM%), phase angle, fat free mass percentage (FFM%) and body cell mass percentage (BCM%), while there was a positive correlation between osteoprotegerin and fat mass percentage (FM%). We demonstrated higher OPG serum levels in OSA patients with cardiovascular diseases than in those without comorbidities (4.01 vs 3.46pmol/l, p<0.05).
ConclusionOur findings, combined with previous observations in other diseases, suggest that elevated OPG serum levels together with selected body composition parameters may be helpful in identifying OSA patients with increased cardiovascular risk.
Osteoprotegerin (OPG) belongs to the TNF (tumor necrosis factor) receptor family. This soluble glycoprotein is a key regulator in bone turnover.1 OPG inhibits osteoclast differentiation and bone resorption.2,3 However OPG expression has been observed not only in bone but also in many other human tissues, including endothelial and smooth vascular cells.2 Osteoprotegerin is a decoy receptor for two important ligands: RANKL (receptor activator of nuclear factor kappa B ligand) and TRAIL (TNF-related apoptosis-inducing ligand). OPG prevents TRAIL-induced apoptosis and inhibits nuclear factor kappa B, which plays a role in regulating inflammation, innate immunity and the vascular system.1,2,4 The presence of OPG was reported in atherosclerotic plaque5 and many authors treat OPG as a mediator of vascular calcification, which is a key part of the atherosclerotic process.1,2,4 In addition, studies have shown that OPG is involved in the development of many cardiovascular diseases and their complications.2,6,7
Bioelectrical impedance analysis (BIA) is a reliable, non-invasive and effective technique for measuring body composition. The principle of BIA is to determine the electric impedance of an electrical current passing through the body. BIA analysis uses the changes in electric current flow through the body depending on its composition and the electrical resistance of the different tissues.8,9 This body composition measurement method was evaluated in different groups of patients including healthy subjects, COPD patients and cancer patients.10,11
The aim of this study was to evaluate the correlations between osteoprotegerin serum levels and body composition parameters in sleep apnea patients and their influence on cardiovascular risk.
Materials and methodsPatientsA total of 125 patients with newly diagnosed obstructive sleep apnea (OSA) syndrome were enrolled in the study. The group examined comprised 91 males and 34 females with a mean age of 54.48±8.81 years. The majority of patients had severe OSA with mean AHI (apnea hypopnea index) of 33.16±20.44/h. Almost all subjects were overweight or obese with mean BMI (body mass index) 33.76±7.18. Many cardiovascular co-morbidities were observed in the OSA patients: 82 had hypertension, 31 diabetes and 25 ischemic heart disease. We divided the OSA patients into two subgroups: those with cardiovascular diseases and those without. The first group (n=89) included patients with one or more cardiovascular diseases, only 36 of the patients presented OSA without any other disease.
A control group was composed of 59 healthy subjects, including 29 females. The mean age was 51.27±12.97 years and the mean BMI was 29.47±5.42.
PolygraphyAll subjects underwent a nocturnal respiratory polygraphy using a Grass Aura Lite PSG (Warwick, USA). All examinations were performed in hospital. There following channels were used in our study: nasal pressure airflow signal, pulse, saturation, electrocardiogram (ECG), thorax and abdomen movements, leg movements, snoring and body position. The following parameters were evaluated during 8h of nocturnal sleep: AHI, desaturation index (DI), mean and minimum SaO2 at the end of sleep apnea/hypopnea episodes. Apnea was defined as a cessation of airflow for more than 10s12 and hypopnea as a reduction in airflow by at least 30% of its value during wakefulness for at least 10s followed by a 4% or greater decrease in oxyhemoglobin saturation.13 An oxygen desaturation event was detected when oxygen saturation fell by at least 4%.
Body compositionAll subjects underwent bioelectrical impedance analysis (BIA) with a single-frequency bioimpedance analyzer (Model BIA 101, AKERN-RJL, Italy). Measurements were taken in the morning, while the patients lay comfortably with their limbs abducted. Current injection electrodes were placed below the phalangeal–metacarpal joint in the middle of the right hand on the dorsal side and below the metatarsal arch on the upper side of the right foot. Detector electrodes were placed on the posterior side of the right wrist and ventrally across the right medial ankle bone. Body composition was determined by injecting 800μA and 50kHz of alternating sinusoidal current using standard tetrapolar technique. Body composition parameters were calculated using the BODYGRAM 1.31 software.
OsteoprotegerinBlood samples were collected from fasting subjects in the morning. After centrifugation for 10min at 1467 RCF, the serum was extracted and stored at −80°C. Osteoprotegerin serum levels were measured using the enzyme-linked immunosorbent assay (ELISA) method and the following kit: Human Osteoprotegerin (R&D Systems, Minneapolis, USA). The tests were performed according to the manufacturer's specifications. The ELISA microplate reader from MRXe Dynex Technologies (Chantilly, USA) was used.
The following biochemical parameters were also measured in the blood serum sample: CRP, total cholesterol, LDL cholesterol, HDL cholesterol, and triglycerides.
Statistical analysisStatistical analysis was performed using the CSS Statistica software for Windows (version 5.0). Spearman's r correlation coefficient was used to assess the relationship between two variables and the Mann–Whitney U test to compare values between the two groups. Differences between samples were considered significant at p<0.05.
This work has been approved by the institution's relevant ethics committees: the Commission of Bioethics at Wroclaw Medical University. All patients gave their written informed consent to participate in the study.
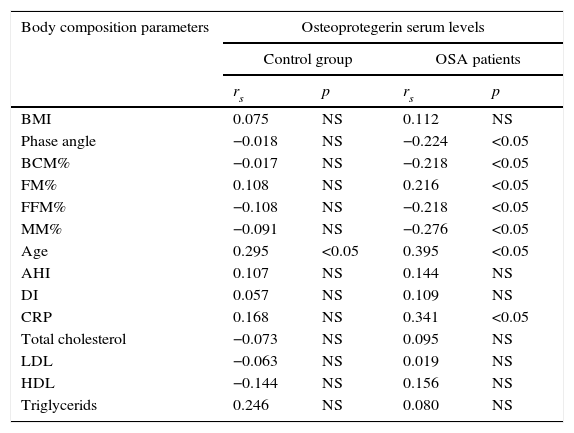
ResultsMany correlations between osteoprotegerin serum levels and body composition parameters were observed in OSA patients. Osteoprotegerin correlated negatively with muscle mass percentage (MM%), phase angle, fat free mass percentage (FFM%) and body cell mass percentage (BCM%), while higher osteoprotegerin was associated with higher fat mass percentage (FM%). In the control group we were unable to find any correlations between body composition parameters and osteoprotegerin serum levels (Table 1).
Correlation coefficients between osteoprotegerin serum levels and selected body composition parameters in OSA patients and control group.
| Body composition parameters | Osteoprotegerin serum levels | |||
|---|---|---|---|---|
| Control group | OSA patients | |||
| rs | p | rs | p | |
| BMI | 0.075 | NS | 0.112 | NS |
| Phase angle | −0.018 | NS | −0.224 | <0.05 |
| BCM% | −0.017 | NS | −0.218 | <0.05 |
| FM% | 0.108 | NS | 0.216 | <0.05 |
| FFM% | −0.108 | NS | −0.218 | <0.05 |
| MM% | −0.091 | NS | −0.276 | <0.05 |
| Age | 0.295 | <0.05 | 0.395 | <0.05 |
| AHI | 0.107 | NS | 0.144 | NS |
| DI | 0.057 | NS | 0.109 | NS |
| CRP | 0.168 | NS | 0.341 | <0.05 |
| Total cholesterol | −0.073 | NS | 0.095 | NS |
| LDL | −0.063 | NS | 0.019 | NS |
| HDL | −0.144 | NS | 0.156 | NS |
| Triglycerids | 0.246 | NS | 0.080 | NS |
Higher OPG serum levels were observed in OSA patients with cardiovascular diseases compared with those with OSA without co-morbidities (4.01 vs 3.46pmol/l, p<0.05) (Fig. 1). In addition, in the group of OSA patients with cardiovascular diseases, phase angle (5.55 vs 5.98 p<0.05) and MM% (41.42 vs 45.15 p<0.05) were lower than in OSA patients without co-morbidities.
There were no statistically significant differences in osteoprotegerin serum levels between OSA patients and those in the control group (3.85 vs 3.93pmol/l) and we did not observe any correlations between osteoprotegerin and OSA parameters such as AHI, DI or mean saturation.
We demonstrated a positive relationship between age and osteoprotegerin in the control group (rs=0.295; p<0.05) and in OSA patients (rs=0.395; p<0.05). Osteoprotegerin correlated positively with CRP (C-reactive protein) in OSA patients (rs=0.341; p<0.05), but not in the control group. No significant correlations were found between OPG and total cholesterol, LDL, HDL or triglycerides in both groups: OSA patients and the control group.
DiscussionOur study showed many correlations between osteoprotegerin serum levels and body composition parameters in OSA patients. In the literature, we were not able to find any previous studies on osteoprotegerin serum levels in OSA patients and correlations between osteoprotegerin and body composition parameters in this syndrome, so we were only able to use base experimental studies and observations made for other diseases.
Einvik et al. examined OPG in patients at high risk of obstructive sleep apnea, however these authors concentrated on associations between depressive syndrome and inflammatory markers. They showed that CRP was associated with depressive symptoms, but did not observe any correlation between depression and OPG or adiponectin.14
In our study, in OSA patients the osteoprotegerin correlated negatively with phase angle, FFM%, BCM% and MM% and we also found a positive correlation between osteoprotegerin and FM%. However OPG did not correlate with BMI and the relationship between obesity and OPG is disputable. Gannage-Yared et al. were unable to find any correlation between OPG and BMI, and did not demonstrate differences between OPG levels in obese and non-obese patients.15 In contrast, Ashley et al. observed decreased OPG levels in obese patients and negative correlation with BMI.16
Previous findings indicate the relationship between OPG and adipose tissue. After evaluation of OPG expression in the fatty tissue, Pobeha et al. suggested that this expression is related to osteoporosis in COPD patients and OPG acts as mediator between fat mass and bone mineral density.17 In addition, observations made by Frederiksen et al. during testosterone therapy suggested that decreased OPG levels could be associated with changes in fat tissue distribution.18 Our findings that OPG correlated positively with fatty tissue percentage are in contrast to observations made by Eagan et al. In stable COPD patients, these authors showed that a higher FMI (fat mass index) was associated with lower osteoprotegerin plasma levels, whereas FFMI was unrelated to osteoprotegerin.19
It is interesting that correlations between osteoprotegerin and body composition parameters were only demonstrated in OSA patients, but not in the control subjects.
These findings could be connected with affected bone metabolism in OSA patients and our results may suggest that in OSA patients bone turnover increases with obesity. Unfortunately, we did not take any bone density measurements from the patients examined. The influence of OSA on bone density is disputable. Some studies have suggested an increased risk of osteoporosis in OSA patients connected with increased bone resorption and suppressed bone formation. Uzkeser et al. demonstrated decreased levels of BMD at femoral and lumbar sites in OSA patients20 while other authors showed an increase in the bone resorption marker, reversed by CPAP treatment in about 50% of patients.21 Hypoxia and oxidative stress in particular, which are common in OSA, have a negative effect on bone turnover.21 However other authors present conflicting results. Sforza et al. studied a very large group (833 persons) and found higher femoral and spinal BMD in OSA patients and positive associations between T-scores and AHI and DI.22
However OPG could be treated as a marker of vascular damage and correlations between osteoprotegerin and body composition could reflect their influence on cardiovascular risk, especially given that in OSA patients with cardiovascular diseases we demonstrated higher OPG serum levels and lower phase angle and MM%. Our results connecting increased OPG with a higher cardiovascular risk agree with many previous findings.7,23 Blazquez-Medela et al. showed in a large group of patients (n=191), that OPG could be treated as an indicator of diabetes and hypertension associated vascular pathologies. OPG levels were higher in hypertensive patients with retinopathy, in patients with three or more damaged target organs (heart, vessels, kidney) and in patients with a history of schaemic cardiopathy episodes.23 In addition, it has been demonstrated that, OPG serum levels are higher in patients with carotid plaque than in those without.24
A negative correlation with phase angle may also confirm that OPG could indicate an increased risk of cardiovascular diseases in OSA patients. Phase angle provides information about cell membrane function, hydration and body cell mass.9,25 It is a marker of training status and decreased phase angle could indicate impaired nutritional and functional status.9,25 Some studies demonstrated that lower phase angle could be recognized as a new marker of increased cardiovascular risk.26
Our observations that OPG correlated positively with age15,23 and CRP15 are in agreement with some previous studies, especially given that OPG is treated as inflammatory marker. We were unable to find any correlations between OPG and cholesterol fractions, as observed by Gannage-Yared et al.15
We are aware that our study has several limitations. First of all, the majority of OSA patients examined had cardiovascular diseases (CVD). This proportion was connected with a close association between OSA and cardiovascular morbidity.27–29 Some authors have even indicated that OSA should be treated as an independent risk factor for CVD.27 However a bigger group of OSA patients without CVD could be helpful in providing better identification of OSA patients with or without lower cardiovascular risk, which could influence therapeutic approaches in the future. In addition, this group of OSA patients is not well enough described, because the majority of studies concentrate on OSA patients with CVD, as we did. Many mechanisms are connected with higher CVD incidence in OSA, including increased sympathetic activity, oxidative stress, systemic inflammation, abnormal coagulation, endothelial dysfunction and metabolic dysregulation.27–29 That is why only one parameter of low OPG serum level is not enough to exclude cardiovascular risk in OSA. Elevated OPG together with some body composition parameters may only suggest increased cardiovascular risk, but more studies are needed to explain these relationships.
ConclusionOur findings, combined with previous observations in other diseases, suggest that elevated OPG serum levels together with selected body composition parameters may be helpful in identifying OSA patients with increased cardiovascular risk.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
AuthorshipMK, PP and IP collected information about patients and performed examinations. MK performed BIA measurements, statistical analysis and drafted manuscript. RJ coordinated the study and made improvements in the manuscript. All authors read and approved the final manuscript.