A tertiary care hospital in North India.
ObjectiveTuberculosis (TB) remains a major public health problem in developing countries. The diagnosis of tuberculosis is still challenging in primary care settings in endemic countries like India. WHO has endorsed loop mediated isothermal amplification assay (LAMP) for TB as a replacement for smear microscopy for peripheral settings, however, more data is required to establish the specificity of this modality for the diagnosis of TB. In this study we aim to determine the diagnostic accuracy of the TB-LAMP assay in pulmonary tuberculosis.
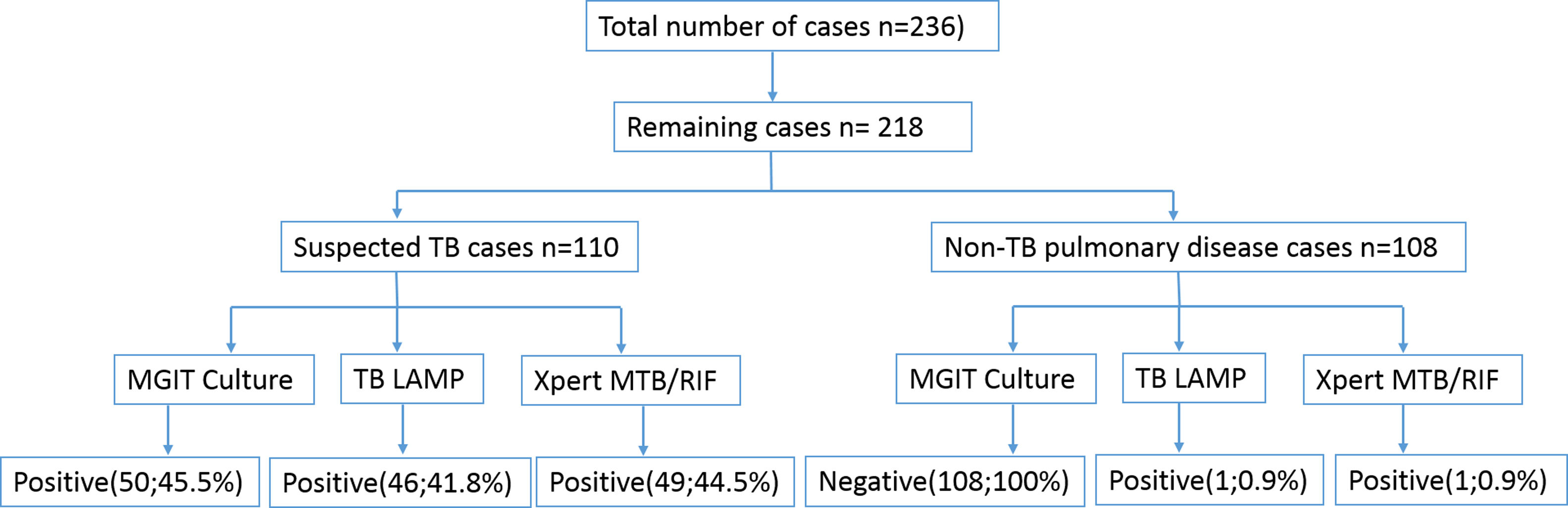
DesignA total of 236 patients (117 cases suspected of TB and 119 patients with non-TB pulmonary disease) were enrolled between February to July, 2018. Microbiological workups consisting of mycobacterial smear microscopy, culture, Xpert MTB/Rif and TB-LAMP were performed.
ResultsFrom 236 samples, 18 (7.6%) were excluded from the study. TB-LAMP and Xpert MTB/RIF were positive in 46 (21.1%) and 49 (22.5%) of the samples, respectively. The sensitivity of Xpert MTB/RIF and TB-LAMP, when culture was taken as a reference standard, was 90% (95%CI: 78.2-96.7) and 82% (95%CI: 68.6...91.4), respectively. The specificity, positive predictive value (PPV), and negative predictive value (NPV) of TB-LAMP assay were 96.8% (95%CI: 92.8...98.9), 89.1% (95%CI: 77.4...95.2), and 94.4% (95%CI: 90.4...96.5), respectively.
ConclusionThe TB-LAMP assay showed a good specificity and sensitivity for detection ofM. tuberculosis in adults, however, for programmatic implementation, more studies are required to be conducted at peripheral level healthcare settings.
Tuberculosis (TB) poses a colossal challenge for national tuberculosis control programmes owing to high morbidity and mortality. In 2018, WHO projected a total of ten million new cases, with a mortality of 1.5 million cases across the globe. Thirty endemic countries account for 87% of the TB cases, of which India contributes a total of 2,155,894 notified cases, in spite of stringent elimination programs in place.1 One of the major contributing factor is high prevalence of affected but hidden cases. Many cases remain undiagnosed due to the inaccessibility to diagnostic tests at peripheral level or due to poor performance of the available tests. The gold standard for the diagnosis of TB remains the culture. However, it is labor-intensive and the results are available quite late. Moreover, the primary test in most of the settings is smear microscopy with low reproducibility and sensitivity as low as 50...70%.2,3 Nucleic acid amplification tests (NAATs) are useful for rapid diagnosis of the cases, however, the cost and pre-requisite of sophisticated laboratory settings are the main impediments in the peripheral and rural areas. To address this limitation, World Health Organization (WHO) has endorsed cartridge based NAATs (CB-NAAT), the Xpert MTB/RIF assay, for uniformity in diagnosis and detection of drug resistance to rifampicin.4 Xpert MTB/RIF assay is easy with a shorter turnaround time (TAT). The overall sensitivity and specificity of Xpert MTB/RIF assay in respiratory samples has been reported to be 88% and 98%, respectively. However, the sensitivity and specificity for diagnosis of extra-pulmonary cases was lower but not inferior, when compared to culture as a reference standard. Recently, the newer generation Xpert, i.e. Xpert MTB/RIF Ultra has also been endorsed by WHO with a higher sensitivity (3...5% more than Xpert MTB/RIF assay) but decreased specificity.5
Another molecular assay endorsed by WHO as an alternative to smear microscopy for diagnosis of pulmonary TB in symptomatic adults is Tuberculosis- Loop Mediated Isothermal Amplification (TB-LAMP) assay, which has shown a pooled sensitivity of 78% in clinical validation by WHO-FIND. The endorsement of TB-LAMP is another significant step by WHO for accomplishing the goal of ..úEnd TB..Ñ strategy. TB-LAMP is comparatively easier, having strand-displacement along with replication activity, does not require stringent temperature conditions for the reaction, is less labor-intensive, has a higher specificity and sensitivity compared to smear microscopy of 69-100% and has a shorter TAT of one hour.6 The validation has been based upon studies conducted in limited settings, and needs more extensive research before implementation of this test at the peripheral level. All the available studies have been conducted on the TB suspect samples without including any control arm. Hence, the present study was designed to determine the diagnostic accuracy of TB-LAMP assay in TB suspects as well as in patients with pulmonary diseases other than TB.
MethodologyStudy settingThe study was carried out in the Department of Medical Microbiology, Postgraduate Institute of Medical education and Research (PGIMER), Chandigarh, India. The laboratory is accredited by NABL:ISO and Central TB Division, India, for routine diagnosis and drug resistance of tuberculosis. The sputum samples from pulmonary diseases other than TB were collected from patients attending the outpatient department of Pulmonary Medicine, PGIMER, Chandigarh. The sputum samples of suspected TB cases were received in the laboratory from different directly observed treatment, short-course (DOTS) centers under Revised National Tuberculosis Control Program (RNTCP) program in Chandigarh. Ethical clearance was taken from Institute Ethics Committee, PGIMER, Chandigarh.
Clinical samplesA total of 236 patients were enrolled from February to July, 2018. Of the 236 patients, 117 were suspected TB cases and 119 patients had pulmonary disease other than TB. One sputum sample from each patient was taken and transferred to the laboratory immediately. Microbiological investigations smear microscopy, mycobacterial culture, Xpert MTB/RIF and TB-LAMP were performed. The demographic details of all the patients were noted from RNTCP request forms and the patient...s proforma.
Smear and mycobacterial cultureThe smears were prepared directly from the sputum samples. Ziehl-Neelsen(ZN) staining was performed and the smears were reported as per RNTCP guidelines. Subsequently, the samples were decontaminated by NALC-NaOH method and 500..L of the processed sample was inoculated in the mycobacterial growth indicator tube (MGIT).7 The tubes were placed in the MGIT 960 instrument (BD, USA) and incubated for 42 days. The positive tubes were confirmed for M. tuberculosis using SD bioline MPT64 Ag kit (Abbott, USA).
Xpert MTB/RIF assayThe Xpert MTB/RIF assay (Cepheid, USA) was performed as per the manufacturer...s instructions. The buffer was mixed in double amount with the sample and incubated at room temperature for 15min. Subsequently, the sample was transferred into cartridge and placed in Xpert machine. The results were interpreted either as M. tuberculosis detected with or without rifampicin resistance, or no target detected.8
TB-LAMP assayThe TB LAMP assay was performed using Loopamp MTBC Detection Kit (Eiken, Japan). The sample (60..L) was transferred into the heating tube and incubated at 95..C for 5min. Then, the heating tube was screw-capped with absorbent tube, mixed and transferred into reaction mix tubes. The reaction mix tube was incubated at 65..C and the final results, in terms of fluorescence, were interpreted.9
Sensitivity and specificity calculationThe M. tuberculosis culture was taken as a reference standard for the calculation of sensitivity, specificity, positive predicted value (PPV) and negative predicted value (NPV). All the parameters were calculated using Medcalc online software.
ResultsOf the 236 samples, 17 samples showed culture contamination and one sample had invalid Xpert result, so 18 (7.6%) samples were excluded from the study. Of the remaining 218 samples, there were 128 (58.7%) males and 90 (41.3%) females. The age of the patients was between 18 to 80 years, with a median of 47 years. A total of 110 (50.4%) samples were from suspected TB patients and 108 (49.5%) from pulmonary disease other than TB. The patients of pulmonary disease other than TB included patients with carcinoma (30, 13.7%), allergic bronchopulmonary aspergillosis (8, 3.7%), asthma (11, 5%), bronchiectasis (13, 5.9%), chronic obstructive pulmonary disease (12, 5.5%), chronic pulmonary aspergillosis (9, 4.1%), cough and chest pain (7, 3.2%), shortness of breath (4.1%), and others (9, 4.1%; allergic rhinitis, hilar mass, primary lung mass, metastatic lesions, fibrosis, sarcoidosis, GERD, pneumothorax, and seasonal allergy). In patients with suspected TB, the culture, Xpert MTB/RIF assay and TB-LAMP was positive in 50 (22.9%), 49 (22.5%) and 46 (21.1%) samples, respectively (Fig. 1).
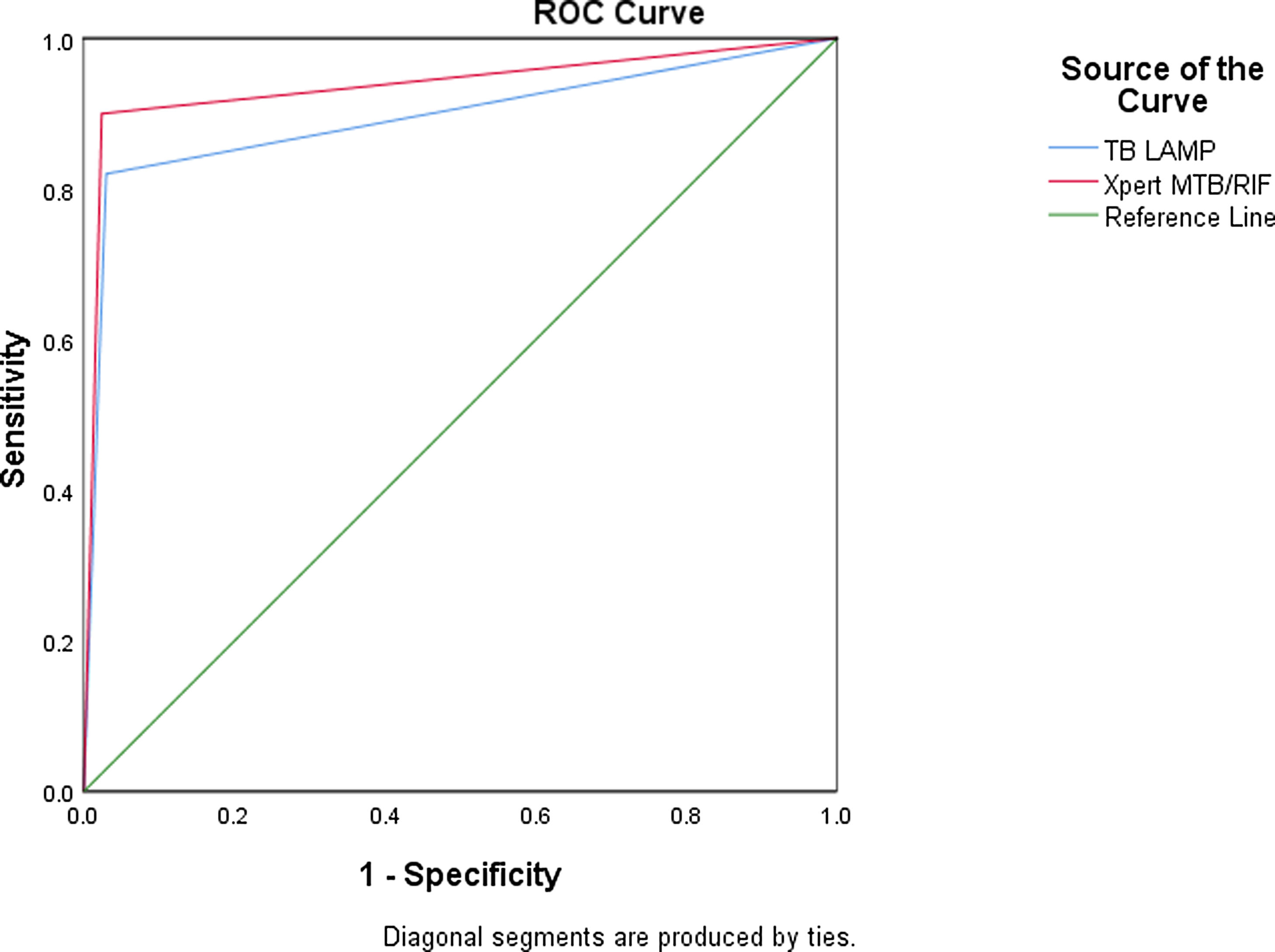
Diagnostic accuracy of TB LAMP and Xpert MTB/RIFThe sensitivity of TB-LAMP, Xpert MTB/RIF and smear was noted to be 82% (95% CI: 68.6-91.4%), 90% (95% CI: 78.2-96.7%) and 64% (95% CI: 49.2-79.1%), respectively, using culture as a reference standard. The specificity, PPV and NPV of TB-LAMP assay was found to be 96.8% (95% CI: 92.8-98.9%), 89.1% (95% CI: 77.4-95.2%) and 94.4% (95% CI: 90.4-96.5%), respectively. On the contrary, the specificity, PPV and NPV of Xpert MTB/RIF was noted to be 97.5% (95% CI: 93.6-99.3%), 91.8% (95% CI: 80.9-96.5%) and 96.8% (95% CI: 93.1- 98.6%), respectively, which is more than TB-LAMP. However, the sensitivity and specificity of TB LAMP and Xpert MTB/RIF in smear negative cases was 55.6% (95% CI: 30.8-78.5%) and 75% (95% CI: 50.9-91.3%) or 97.5% (95% CI: 93.8-99.3%) and 98.1% (95% CI: 94.5-99.6%), respectively. The specificity and NPV of TB-LAMP assay in non-TB cases was found to be 99.1% (95% CI: 94.9- 99.9%) and 100%, respectively (Table 1). The area under curve (AUC) of receiver operating characteristic (ROC) curve for TB-LAMP and Xpert MTB/RIF was 0.895 and 0.938, respectively (Fig. 2).
Diagnostic accuracy of TB LAMP and Xpert MTB/RIF assay in different groups.
| Patient group | Diagnostic assay | Sensitivity % (95% CI) | Specificity% (95% CI) | Positive predictive value % (95% CI) | Negative predictive value % (95% CI) |
|---|---|---|---|---|---|
| Overall | Xpert MTB/RIF | 90 (78.2...96.7) | 97.5 (93.6...99.3) | 91.8 (80.9...96.5) | 96.8 (93.1...98.6) |
| TB LAMP | 82 (68.6...91.4) | 96.8 (92.8...98.9 | 89.1 (77.4...95.2) | 94.4(90.4...96.5) | |
| Smear | 64 (49.2...79.1) | 99.4 (96.5...99.9) | 96.9 (81.8...99.6) | 89.7(85.8...92.7) | |
| Smear Negative cases | Xpert MTB/RIF | 75 (50.9...91.3) | 98.1 (94.5...99.6) | 83.3(61.3...94) | 96.8(93.5...98.5) |
| TB LAMP | 55.6 (30.8...78.5) | 97.5(93.8...99.3) | 71.4(46.6...87.8) | 95.2(92.1...97.1) | |
| Smear Positive cases | Xpert MTB/RIF | 100 (88.8-100) | 98.2 (93.5-99.8) | 93.9 (79.7-98.4) | 100 |
| TB LAMP | 96.8 (83.3...99.9) | 96.4 (91...99) | 88.2 (74.1...95.2) | 99.1 (93.9...99.9) | |
| Non-TB pulmonary disease cases | Xpert MTB/RIF | ... | 99.1% (94.9...99.9%) | ... | 100 |
| TB LAMP | ... | 99.1% (94.9- 99.9%) | ... | 100 |
WHO has endorsed TB-LAMP to strengthen diagnostic testing in peripheral health centers to achieve the WHO ..úEnd TB..Ñ strategy.10,11 The Xpert MTB/RIF assay has been evaluated in many studies across the globe, however, the same does not hold true for TB-LAMP assay, for which very limited studies have been conducted in different geographical regions. In this study, we appraised the sensitivity and specificity of TB LAMP assay in suspected TB as well as non-TB pulmonary disease cases.
In the present study, the overall specificity of TB-LAMP assay was noted to be 96.8%, which is in concordance to other studies that have shown specificity of 96.7...98.7%.12,13 The overall specificity of Xpert MTB/RIF assay in our study was 97.5%, which was higher than the TB-LAMP assay. This is in concordance with other studies, most of which have revealed a specificity of 97.2...99.3%. The sensitivity of TB LAMP assay was 82%, which is comparable to other studies in which have shown sensitivity of 92...100% respectively.9,14...16 On the contrary, the studies conducted in China, India and Vietnam revealed sensitivity as low as 70.6...79.6%.12,17 The overall sensitivity of the TB-LAMP assay was higher than the smear (82% vs. 64%). In smear negative and smear positive TB cases, it was 55.6% and 96.8%, respectively. However, the previous studies had also shown the similar sensitivities in smear negative cases, e.g. Kim et al. and Pham et al. (46.6...58.8%).12,15 The indeterminate results with TB-LAMP were exceptionally rare18 and the same was noted in the present study.
The sensitivity of Xpert MTB/RIF assay was 90% in our study, which was better than TB-LAMP assay. This could be because of the larger volume of sample used in Xpert MTB/RIF as compared to TB-LAMP. A meta-analysis to analyze the diagnostic accuracy of TB-LAMP has concluded that TB-LAMP has moderate sensitivity (77.7%) and high level of specificity (98.1%). The pooled sensitivity and specificity of TB-LAMP is comparable to Xpert MTB/RIF in all, except HIV-positive individuals, in whom the pooled sensitivity falls down to 63.8%. This can be attributed to a high proportion of smear-negativity amongst HIV-positive individuals. The major advantage of Xpert MTB/RIF over TB-LAMP is the detection of rifampicin resistance along with M. tuberculosis. The rifampicin resistance detection by Xpert MTB/RIF helps in better management of RR/MDR-TB where the prevalence of rifampicin resistance is high. However, in this study we did not find any resistance to rifampicin by Xpert MTB/RIF and phenotypic DST was also not performed.
We have included sputum samples from pulmonary illness other than TB to evaluate the specificity of this assay which is major strength of this study. Of these 108 samples, only one was detected as false positive by both TB-LAMP and Xpert MTB/RIF assays. The specificity of this assay in pulmonary disease other than TB has not been previously explored to the best of our knowledge. Other than routine healthcare settings, this assay could also prove to be a useful tool for screening TB in special high-risk settings such as prisons, immigrants and close contact settings. Linhas R et al. discussed the problem of TB screening in immigrants in Portugal and highlighted the importance of the rapid, accurate and economic test for TB screening.18 The limitation of this study lies in small number of samples in both groups (suspected TB and non-TB pulmonary disease) and that phenotypic DST was not performed for rifampicin.
The present study concluded that TB-LAMP could be used as an ancillary test to sputum microscopy or as a follow-up alternative to the same, since it fulfills the WHO criteria of an alternative test.19 Though the sensitivity and specificity in our study was inferior to the Xpert MTB/RIF, there was no significant difference for the same, as has also been reported earlier.20 Moreover, the end-user profile is different for both the tests and the cost effectiveness study conducted by WHO determined low cost per test for TB-LAMP with low operational, budgetary and incremental costs,6 thus facilitating its use as an alternative highly sensitive test in peripheral settings. Therefore, TB-LAMP is a viable alternative to smear microscopy in resource-poor settings with insufficient infrastructure for Xpert MTB/RIF.
Conflicts of interestThe authors have no conflicts of interest to declare.
The financial support from NextGen Invitro Diagnostics Pvt Ltd, India is acknowledged.