It is critical to developing an accurate method for differentiating between malignant and benign solitary pulmonary nodules. This study aimed was to establish a predicting model of lung nodules malignancy in a real-world setting.
MethodsThe authors retrospectively analysed the clinical and computed tomography (CT) data of 121 patients with lung nodules, submitted to percutaneous CT-guided transthoracic biopsy, between 2014 and 2015. Multiple logistic regression was used to screen independent predictors for malignancy and to establish a clinical prediction model to evaluate the probability of malignancy.
ResultsFrom a total of 121 patients, 75 (62%) were men and with a mean age of 64.7 years old. Multivariate logistic regression analysis identified six independent predictors of malignancy: age, gender, smoking status, current extra-pulmonary cancer, air bronchogram and nodule size (p<0.05). The area under the curve (AUC) was 0.8573.
ConclusionsThe prediction model established in this study can be used to assess the probability of malignancy in the Portuguese population, thereby providing help for the diagnosis of lung nodules and the selection of follow-up interventions.
Pulmonary nodules are a common finding on chest computed tomography (CT) and present as a challenge to clinicians. The majority are not malignant and usually have no clinical significance.1.Çô4 Fleischner guidelines5 pertain to the follow-up and management of pulmonary nodules detected incidentally on chest CT. However, their malignant potential is mostly unknown at the time of detection.
Lung cancer is the most significant cause of death from cancer in the world. It is usually diagnosed in an advanced stage, resulting in a 5-year survival rate of 17.4%.6 Its complexity from both histopathological and biological perspectives, perhaps having multiple preneoplastic pathways, poses an enormous challenge for an early diagnosis.7 Thus, to reduce the mortality rate, the ideal would be developing strategies to diagnose lesions in the pre-invasive state. In 2011, the National Lung Screening Trial (NLST) showed a reduction in lung cancer mortality of 26% in men, 39.Çô61% in women and 20% globally, for annual screening with low-dose CT scanning, compared with no screening or with chest radiography.1 The recent publication of the Dutch-Belgian Randomized Lung Cancer Screening (Nelson) Trial, a population-based randomized controlled trial, showed that volume low-dose CT screening had lower lung cancer mortality than no screening, in high-risk patients.8 However, the main challenge in CT screening is the high prevalence of pulmonary nodules and the relatively low incidence of lung cancer.1.Çô5 Several studies have shown that the most important predictors of malignancy for pulmonary nodules include size, appearance and growth rate (volume doubling time <400 days), patient's age, smoking and extrapulmonary tumour history.9.Çô15 Although those factors are supported by clinical experience, they do not seem to be enough to choose a course of action.5,10,12,16,17
Sometimes the question is raised: what is the best clinical guidance after identification of a pulmonary nodule? Percutaneous CT-guided transthoracic biopsy is frequently used for the diagnosis of lung nodules, particularly for peripheral or bronchoscopic inaccessible lung lesions.18,19 Although an effective approach in experienced hands, it has limitations with smaller nodules and ground-glass lesions and, a considerable complication rate (pneumothorax range for 4.Çô40% and 1.Çô7% for haemoptysis).18,20.Çô22 On the other hand, choosing a follow-up strategy increases stress and exposure to radiation from numerous CT scans and allows possibly malignant nodules to evolve, delaying cancer diagnosis and treatment.9,23 Consequently, clinicians must carefully weigh up whether the risk of cancer of a lung nodule justifies the potential harm of a biopsy.
ObjectivesIn this paper, the authors aimed to define which clinical and radiological characteristics could suggest malignancy, in a real-world setting, and therefore better correlate with the decision for a biopsy. Finally, the authors built a model to assist in the decision-making process for an invasive diagnosis.
MethodsStudy populationStudy cohort included patients who underwent percutaneous CT-guided transthoracic biopsy for one year, at Centro Hospitalar Universit.írio de S.úo Jo.úo (Porto, Portugal). Only cases where the biopsy target was less than 3cm diameters in initial CT evaluation were included. Patients with a clinical record of interstitial lung disease were excluded. Written informed consent, before the chest CT biopsy, was obtained from all patients.
The study was approved by Centro Hospitalar Universit.írio de S.úo Jo.úo Ethics Committee.
Data collectionClinical and socio-demographic data were collected from patients.ÇÖ electronic records. The data included age, sex, smoking status (non-smoker, active smoker and former smoker), symptoms at detection and history of tuberculosis. Cancer history was also considered, divided into pulmonary and extra-pulmonary tumours, and subsequently into current or previous illness. Other medical backgrounds, like chronic obstructive pulmonary disease, bronchiectasis, obstructive sleep apnoea syndrome, pulmonary aspergillosis, was also recorded. Later, the histologic result was documented.
Chest CT analysisChest CT-scan were obtained from the hospital's electronic records and analyzed by two Radiology assistants with more than 15 years of experience. The biopsy CT-scan was assessed, retrospectively, for tumour characteristics by a first Radiologist, who was blinded to the clinical and histological findings. The cases were later reviewed and approved by a second Radiologist. Radiological features recorded included the tumour shape (round, ovoid, bilobed or irregular), sphericity and attenuation (pure ground glass, semisolid or solid), tumour location (central or peripheral), margins (smooth, spiculated or lobulated), presence of internal air bronchogram, cavitation, single nodule and pleural contact. The size was obtained from the first CT available. Measurements were made to the nearest centimetre using manually placed computer electronic callipers considering the nodule biggest axis.
Statistical analysisCategorical variables are presented as frequencies and percentages and were compared with the use of the Chi-square test. Continuous variables are presented as means and standard deviations and were compared with the use of the t-test. The interaction of these variables with the biopsy result was expressed as risk ratios (RRs). A logistic regression model was constructed to assess the association between the outcome (benign/malignant) and the pre-biopsy characteristics. Individual malignancy probability could be obtained by getting the sum of the products of the coefficient of each independent variable (..1.êÆn) included in the logistic model and their code (X1.êÆn) .Çô according to the table score .Çô and replacing it in the formula:
Odds ratio (OR) and 95% confidence interval (CI) were calculated for the model variables. The significance level was set at p<0.05 (two-sided). IBM SPSS Statistics 24, STATA Statistical Data Analysis 9.0 and BiostatXL MIX 2.0 were used to compute all these estimates.
ResultsDuring the study period, 121 patients were eligible. The mean age was 64.7..12.3 years, and 75 (62%) of the patients were male. The majority (84.5%) were discovered by accident. Table 1 shows the patients.ÇÖ demographic and clinical characteristics. Malignant nodules were observed in sixty-four (53%) patients. The majority of those were lung adenocarcinoma (n=35, 54.7%). Other malignancies included carcinoid tumour (n=11, 17.2%), extrathoracic tumours (n=7, 11%), squamous-cell carcinoma (n=4, 6.3%), large-cell carcinoma (n=2, 3.1%), small-cell lung carcinoma (n=2, 3.1%), lymphoma (n=2, 3.1%) and adenosquamous carcinoma (n=1, 1.6%). Twenty-three (40.4%) patients, with benign lung nodules, had no specific diagnosis (histology with no signs of malignancy), and the remainder included chondroid hamartoma (n=13, 22.8%), benign neoplasms (n=11, 19.1%) and infectious process (n=10, 17.5%).
Clinical data description and relationship between these and final biopsy results; TB .Çô tuberculosis.
| Population characteristics | Total, n (%) | Malignant Nodule, n (%) | Benign Nodule, n (%) | Missing, n (%) | p Value |
|---|---|---|---|---|---|
| Age | 0.037 | ||||
| <70 years | 73 (60.3) | 33 (45.2) | 40 (54.8) | ||
| .ëÑ70 years | 48 (39.7) | 31 (64.6) | 17 (35.4) | ||
| Gender | 0.531 | ||||
| Male | 75 (62) | 38 (50.7) | 37 (49.3) | ||
| Female | 46 (38) | 26 (56.5) | 20 (43.5) | ||
| Smoking status | 5 (4.1) | 0.505 | |||
| Not smoker | 39 (33.6) | 21 (53.8) | 18 (46.2) | ||
| Current smoker | 31 (26.7) | 14 (45.2) | 17 (54.8) | ||
| Former smoker | 46 (39.7) | 27 (58.7) | 19 (41.3) | ||
| Current extra-pulmonary cancer history | 16 (13.9) | 13 (81.3) | 3 (18.8) | 0.015 | |
| Pulmonary cancer history | 5 (4.3) | 4 (80.0) | 1 (20.0) | 6 (5.0) | 0.217 |
| Previous TB | 7 (6.0) | 3 (42.9) | 4 (57.1) | 5 (4.1) | 0.562 |
| Accidental finding | 98 (84.5) | 53 (54.1) | 45 (45.9) | 5 (4.1) | 0.452 |
| Central localization | 11 (9.1) | 10 (90.9) | 1 (9.1) | 0.008 | |
| Sphericity | 112 (92.6) | 61 (54.5) | 51 (45.5) | 0.222 | |
| Margins | |||||
| Smooth | 108 (89.3) | 55 (50.9) | 53 (49.1) | 0.212 | |
| Lobulated | 55 (45.5) | 36 (65.5) | 19 (34.5) | 0.012 | |
| Spiculated | 64 (52.9) | 40 (62.5) | 24 (37.5) | 0.025 | |
| Calcification | 3 (2.5) | 1(33.3) | 2 (66.6) | 0.492 | |
| Air bronchogram | 89 (73.6) | 53 (59.6) | 36 (40.4) | 0.014 | |
| Cavitation | 9 (7.4) | 5(55.6) | 4 (44.4) | 0.869 | |
| Single nodule | 66 (54.5) | 39(59.1) | 27 (40.9) | 0.135 | |
| Pleural contact | 35 (28.9) | 26 (74.3) | 9 (25.7) | 0.003 | |
| Size, mean (range) mm | 14.21 (3.Çô29) | 16.45 (5.Çô29) | 11.68 (3.Çô23) | <0.001 | |
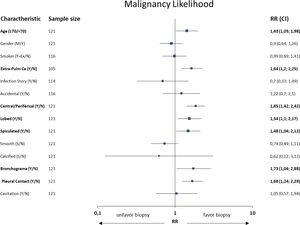
Patients with malignant nodules were significantly older than patients with benign nodules (.ëÑ70 years: 64.6% vs. 35.4%, p=0.037, RR=1.43) and more likely to have current extra-pulmonary cancer (81.3% vs. 18.8%; p=0.015, RR=1.64); however, these association were not identified in the previous history of extra-pulmonary cancer. Radiological characteristics were identified with significant associations between malignancy and a central location (p=0.008, RR=1.85), lobulated (p=0.012, RR=1.54) and spiculated (p=0.025, RR=1.48) margins, air bronchogram (p=0.014, RR=1.73), pleural contact (p=0.003, RR=1.68), size (p<0.001). Neither the shape, predominant margins, calcification, single nodule nor attenuation differences were statistically associated with a malignant nodule. Table 1 and Fig. 2 show the interaction between some of the patients.ÇÖ clinical and radiological characteristics and the final biopsy result.
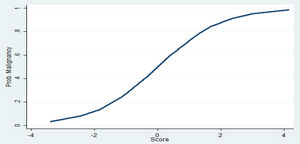
Clinical and radiological characteristics were then used to build a logistic model to predict the probability of nodule malignancy. Of those, age, gender, smoking status, current extra-pulmonary cancer, nodule size and presence of air bronchogram were independent risk factors for malignancy (Table 2). Patients .ëÑ70 years old (OR 4.77; 95% CI: 1.65.Çô13.76) and female patients (OR 6.51; 95%CI: 1.50.Çô28.10), for instance, were more likely to have malignant nodules. The likelihood of malignancy also increased with every 1-mm increase in diameter (OR 1.25; 95% CI: 1.12.Çô1.38). The clinical prediction model is described as follow: .êÆ8.61+(1.56.ùAge Category)+(1.87.ùGender)+(1.26.ùSmoking Status)+(1.28.ùCurrent ExtraPulmonary Cancer)+(1.27.ùAir Bronchogram)+(0.22.ùNodule Size).ÇôFig. 1 and Table 3.
Multivariate regression analysis of independent risk factors for malignancy.
| Variables | Odds ratio | p Value | 95% Confidence interval |
|---|---|---|---|
| Age <70/.ëÑ70 years | 4.77 | 0.004 | 1.65.Çô13.76 |
| Gender | 6.51 | 0.012 | 1.51.Çô28.11 |
| Smoking status | 3.53 | 0.003 | 1.52.Çô8.17 |
| Size | 1.25 | <0.001 | 1.12.Çô1.38 |
| Air bronchogram | 3.59 | 0.030 | 1.13.Çô11.45 |
| Current extra-pulmonary cancer | 8.94 | 0.009 | 1.72.Çô46.44 |
Thus, for example, the probability of a 60 year-old male patient, active smoker, with no current extra-pulmonary cancer and a 10mm nodule with air bronchogram, having a malignant nodule could be computed by: .êÆ8.61+(1.56.ù0)+(1.87.ù1)+(1.26.ù1)+(1.28.ù0)+(1.27.ù1)+(0.22.ù10)=.êÆ2.01 which indicates a low probability of malignancy -2.5 to 27%. However, a .ëÑ70 year-old female patient, former smoker, with no current extra-pulmonary cancer and with a 15mm nodule with air bronchogram: .êÆ8.61+(1.56.ù1)+(1.87.ù2)+(1.26.ù0)+(1.28.ù0)+(1.27.ù1)+(0.22.ù15)=6.04 has a malignancy probability of 87 to 99%.
The accuracy of the final model was good, with an area under the curve (AUC) of 0.8573. Its sensitivity was 78%, specificity was 85%, the positive predictive value was 85.2%, and the negative predictive value was 78% (Supplementary material). The area under ROC curve of our model [AUC=0.8573 (95% CI, 0.778.Çô0.919)] was significantly higher than the Brock model [AUC=0.7384 (95% CI, 0.646.Çô0.813)], p=0.005. ROC curve of our proposed model and the Brock model are displayed in Fig. 3.
DiscussionThe authors investigated a sample of 121 Portuguese patients to establish which characteristics would identify a malignant lung nodule. The final model identified four clinical indicators (age, gender, smoking status and current extra-pulmonary cancer) and two imaging indices (maximum nodule diameter and presence of air bronchogram) relevant to estimating the probability of malignancy and help guide follow-up decision. For the most part, these findings were consistent with previous studies. Older age,10,16,24.Çô26 gender,27 smoking history10,24.Çô27 and maximum nodule diameter10,16,24.Çô26,28 were already referred to as lung cancer predictors. Furthermore, this study identified air bronchogram and current extra-pulmonary cancer as relevant in the decision-making process. In contrast to the BTS guidelines,15 which recommends the application of the Brock model,17 our study did not find the presence of spiculation or a predominant spiculated margin and part-solid nodules to be relevant in estimating the probability of malignancy.
The need to distinguish benign and malignant nodules makes the clinical management of pulmonary nodules challenging. The first recommendations, as a standard practice, regarded all noncalcified pulmonary nodules as potentially malignant lesions, requiring follow-up CT screening, until proven stable, for a period of 2 years.29,30 Later, the 2017 Fleischner Society Guidelines5 increased the size threshold for routine follow-up of solid nodules to 6mm, because of data from several screening trials which indicated that the risk of lung cancer in nodules <6mm is considerably less than 1%, even in patients at high-risk. However, these individuals may warrant follow-up at 12 months, if they have a suspicious morphology, upper lobe location, or both. Solid nodules measuring 6.Çô8mm in patients with low clinical risk are recommended for follow-up at 6.Çô12 months, extending to 24 months depending on morphology and if stability is uncertain. In high-risk patients, the initial follow-up examination is at 6.Çô12 months and always extends to 24 months. For nodules larger than 8mm in diameter, both invasive and non-invasive management options are included. Despite these recommendations, some studies31.Çô33 have shown that the management of pulmonary nodules does not always receive follow-up concordant with Fleischner Society Guidelines.
Furthermore, the increasing volume of chest imaging and improved image technology is going to create a burden of patients with nodules that need to be managed.31 Accordingly, there is a growing recognition of the potential utility of risk models to predict lung cancer in patients with pulmonary nodules and allowing more subjects to be monitored with low-dose CT imaging rather than needing invasive procedures. Al-Ameri et al.4 aimed to validate four models in a UK population .Çô three models based on clinical and CT characteristics (Mayo Clinic,25 Veterans Association,10 Brock University17), and a fourth model (Herder34) additionally incorporating 18Fluorine-Fluorodeoxyglucose (FDG) avidity on positron emission tomography-computed tomography (PET.ÇôCT). Both the Mayo (AUC=0.752) and Brock (AUC=0.878) models perform well in routine clinical practice. For small pulmonary nodules, the highest AUC value was seen for the Brock model, although there was no significant difference compared to the Mayo model. For patients who underwent PET.ÇôCT for nodule evaluation, the Herder prediction model had the highest accuracy. Several other prediction models have been created using clinical and radiological criteria to assist clinicians to discriminate malignant from benign nodules. Older age, smoking history, maximum nodule diameter and spiculation in chest CT appear most frequently as predictors of lung cancer in most of the final models.16,24,26,35.Çô37
Overall, our model discriminated well with an excellent overall performance. Its values for discrimination and calibration were comparable to the Brock model17 (recommended in the BTS guidelines). A direct comparison, using our population, is not entirely accurate, as family history of cancer was not collected in our study (in terms of comparison, it was considered absent when applied to the Brock model). Our model classified five malignant nodules with a low likelihood of malignancy, and those had, also, a lower probability calculated by the Bock model (mean..standard deviation: 8.2%..6%). Moreover, forty-three malignant nodules, that had a high or very high probability of malignancy with our model had a mean probability by the Bock model of 33.6%..19%, with more dispersed values (minimum-maximum: 4.6.Çô76.1%). Conversely, forty-five non-malignant nodules classified with low or fair probability of malignancy, also had lower mean probability by the Bock model (10.1%..7.7%). Furthermore, the AUC of our model performed significantly better than the Brock model, demonstrating that, in a real clinical setting, our model had a similar prediction ability to the Brock model. It is crucial to emphasize that the role of a prediction model is to guide intervention; applying it can enable timely diagnosis and treatment of malignant nodules, prevent unnecessary invasive examinations and surgery for benign nodules, but can never substitute the physician's decision.
However, our study has some limitations. A retrospective study, with a sample of patients who had undergone biopsy, may overestimate the prevalence of malignancy. Moreover, it is a geographically limited group, therefore lacking external validation of this model. Some potentially relevant data was not collected, like pack-years, time since quitting smoking, family history of cancer, variables previously indicated as independent factors for malignancy.
In conclusion, a combination of risk factors for malignancy (age, gender, smoking status, current extra-pulmonary cancer, maximum nodule diameter and presence of air bronchogram) can enable accurate differentiation of malignancy from benignancy in lung nodules. To the best of our knowledge, this is the first model pertaining to a Portuguese population and, additionally, with good discrimination, with an AUC value similar to other validated prediction models. This model can help decide the need for a lung biopsy and, thus reducing useless invasive techniques. Although the mathematical models provide an objective basis for judging the character of SPN, we need to emphasize that this prediction model cannot take the place of pathological diagnosis.
Conflicts of interestThe authors have no conflicts of interest to declare.






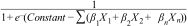
![ROC curve of our proposed model and the Brock model. The area under ROC curve of our model is significantly higher than the Brock model [AUC=0.8573 (95% CI, 0.778.Çô0.919) vs. AUC=0.7384 (95% CI, 0.646.Çô0.813)], p=0.005. ROC curve of our proposed model and the Brock model. The area under ROC curve of our model is significantly higher than the Brock model [AUC=0.8573 (95% CI, 0.778.Çô0.919) vs. AUC=0.7384 (95% CI, 0.646.Çô0.813)], p=0.005.](https://static.elsevier.es/multimedia/25310437/0000002800000006/v1_202211020651/S2531043720301483/v1_202211020651/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w9znTMwFdb/TnkS0koegILxs=)





