Pulmonary tuberculosis (TB) requires an early diagnosis for prompt introduction of treatment and prevention of transmission. Definitive diagnosis is obtained by microbiological culture and identification of Mycobacterium tuberculosis in respiratory specimens, mostly sputum samples.
Materials and methodsRetrospective data analysis of all patients suspected of pulmonary TB that submitted three consecutive sputum samples to the Pulmonology Diagnostic Center (PDC) Laboratory between 2004 and 2013. Extrapulmonary TB cases were excluded. Four microbiological analyses were executed on each specimen: two smears with Ziehl–Neelsen staining, direct and concentrate; and two culture examinations, one in liquid and one in solid medium. Statistical analysis was performed by SPSS.
ResultsA total of 694 patients were enrolled in this study (65% men, mean age 48.5±18.6 years, 97% Portuguese), most of them exhibiting TB-related complaints. Pulmonary TB was diagnosed in 41% of the patients; 54% had non-specific radiological changes and 34% had pulmonary cavitation. The cumulative sensitivity rates of each of the three smears were 24.6%, 27.7% and 28.8% for concentrated samples and 19.3%, 20.4% and 22.5% for direct samples. The cumulative sensitivities of sputum culture were 33.3%, 37.9% and 41.8% for solid medium, and 43.9%, 51.6% and 55.4% for liquid medium. Pondering all forms of microbiological analysis, the cumulative sensitivities of each sample were 51.2%, 59.6% and 63.2%. There was an incremental yield of 8.4% for the second specimen and 3.5% for the third specimen. All sensitivity rates were higher among patients with pulmonary cavitation.
ConclusionsThis study showed an incremental yield with more than one sputum sample. However, overall sensitivity remained low, suggesting a need for new diagnostic strategies and novel and better diagnostic tools.
Tuberculosis (TB) is still associated with a high global burden; there was an estimated 8.6 million new cases and 1.3 million deaths in 2012, most of them occurring in low- and middle-income countries.1
Early diagnosis and immediate treatment are essential to cure this airborne infectious disease and prevent transmission in the community. A definite diagnosis can only be established if Mycobacterium tuberculosis is isolated and identified from respiratory specimens, most frequently expectorated or induced sputum.
Therefore, when pulmonary TB is suspected, standard guidelines recommend that clinical specimens should be collected and submitted for laboratory testing, such as smear microscopy, culture and nucleic acid amplification to increase the detection rate of Mycobacterium tuberculosis.2–5
It has been stated that laboratory analysis should be performed on at least three sputum specimens, collected over consecutive days.6,7 However, there has been some controversy attached to this methodology and some authors find examination of multiple specimens excessive in relation to the yield gain and hard to achieve especially in resource-limited settings.8–13
Culture identification is still the gold standard for diagnosis of pulmonary TB despite the fact that Mycobacterium tuberculosis is a slow growing organism and solid medium culture may take up to 4–8 weeks to provide a positive result. To overcome this limitation, liquid medium culture has emerged as a more sensitive and speedier technique to detect bacilli growth and simultaneously test for drug susceptibility.14–16
In developing countries, sputum smear microscopy is the most commonly used diagnostic test for pulmonary TB. It is a simple technique that quickly identifies acid-fast bacilli (AFB) with a relatively low cost and high positive predictive value, especially when concentrated samples are used. It is important as a tool not only to establish a presumptive diagnosis of pulmonary TB but also to monitor the patient response to anti-TB treatment. In 2009, the WHO recommended that the conventional bright field microscopy using Ziehl–Neelsen (ZN) stain should be replaced by the more sensitive fluorescent light-emitting diode (LED) microscopy, but up to 2012 only two percent of the microscopy centers had this kind of equipment.1,17 Nowadays, many countries, including Portugal, use fluorescent microscopy for sputum microscopy on a routine basis.
Rapid molecular tests are recent diagnostic instruments that can be used to simultaneously test for pulmonary TB and rifampicin resistance with higher sensitivity than sputum smear microscopy and which could replace conventional culture-based drug susceptibility testing.18 However, these groundbreaking tests are more expensive, require appropriately equipped laboratory settings which are still unavailable in many high-prevalence countries and demand a change in the diagnosis paradigm.
Thus, at the present time, ZN smear microscopy and solid or liquid medium cultures remain the diagnostic methods most widely used in the diagnosis of TB. The aim of this study was to analyze the sensitivities of these conventional methods in a Portuguese public health department.
Materials and methodsPatient selectionBetween 2004 and 2013, data from all patients submitted to sputum analysis at the Pulmonology Diagnostic Centre (PDC) laboratory was retrospectively assessed. Only those that had three adequate samples collected in consecutive mornings were included in this study. Non-pulmonary and/or pleural tuberculosis, latent tuberculosis infection (LTBI) and presumed TB based only on clinical grounds were excluded.
Patients were diagnosed with LTBI when they exhibited no symptoms or signs of the disease except for a positive tuberculin skin test and/or a positive interferon-γ release assays (IGRA) test. A diagnosis of tuberculosis was made if Mycobacterium tuberculosis was isolated from respiratory samples or if a histological diagnosis was made. Pulmonary TB diagnosis was excluded when all standard diagnostic procedures, including bacteriologic examination, chest radiograph and occasionally histologic analysis, did not confirm active disease suspicion and also, when during follow-up appointments, there was clinical improvement or establishment of an alternative diagnosis.
Microbiology techniqueSputum collectionAll patients received prior oral and written specific instructions on the correct sputum collection technique. Collection was done at home immediately after waking up and washing the mouth with water, avoiding toothpaste or antiseptic solutions. Samples were collected to a sterile container three days in a row, placed inside a thermal bag at low temperature in the fridge, avoiding overgrowth of other bacteria, and delivered to the laboratory in the fourth day.
Digestion and decontamination procedureTo maximize the mycobacterial yield, contaminated specimens were submitted to a digestion and decontamination procedure with the N-acetyl-l-cysteine-sodium hydroxide (NALC-NaOH) method (BBL® MycoPrepTM Specimen Digestion and Decontamination Kit) prior to culture analysis. NALC-NaOH solution was prepared onsite and immediately added to an equal amount of sputum sample (of 5mL each) in a 50mL centrifuge tube due to the rapid loss of NAC activity when the compound is in solution. The decontamination process included adding a phosphate buffer of 6.8 to the 50mL mark before proceeding to centrifugation. The supernatant fluid was carefully decanted and a small amount of the same buffer added to the sediment. The sediment was resuspended and the suspension used for preparation of concentrated smears and the performance of mycobacterial procedures.
Microscopy staining techniqueZiehl–Neelsen stain (BD™ TB Stain Kit ZN) was used to stain two smears prepared from each sputum sample, one direct (unconcentrated) and one concentrated, according to the Ziehl–Neelsen (hot) acid-fast procedure for early presumptive diagnosis of mycobacterial infection.
The concentrated smear was obtained after a digestion and decontamination procedure with N-acetyl-l-cysteine and sodium hydroxide 2% (BD BBL™ MycoPrep™) to maximize the mycobacterial yield.
Smears made from direct and concentrated sputum were observed on optic microscope (Leitz Laborlux K) with oil immersion lens.
Mycobacterial cultureDigested and decontaminated sputum samples were inoculated into a mycobacteria growth indicator tube (MGIT™) with modified Middlebrook 7H9 broth (BBL™ MGIT™), enrichment growth supplement (BBL™ OADC) and an antibiotic mixture against Gram-negative and Gram-positive bacteria and fungi (BBL™ MGIT™ PANTA™) for the detection and recovery of mycobacteria using the BACTEC™ MGIT™ 960 System. This incubator executes continuous monitoring until there is a positive end to the testing protocol. An instrument positive tube contains approximately 105–106 colony-forming units per milliliter (CFU/mL), detected by fluorescence analysis. Positive cultures were confirmed by smear microscope analysis. Culture that remained negative up to 45 days and showed no visible signs of positivity were removed from the instrument as negatives.
Samples were also incubated into a solid egg-based medium (BBL™ Löwenstein–Jensen Medium) for isolation and cultivation of mycobacteria using a Jouan Incubator at 35±2°C up to 8 weeks, beyond which the result is considered negative.
Species identificationA DNA probe test for in vitro routine (INNO-LiPA MYCOBACTERYA v2 assay) was used for the detection and identification of the genus Mycobaterium and 16 different mycobacterial species from solid culture or, if negative, from liquid culture. This assay is based on nucleotide differences in the 16S–23S ribosomal (rRNA) spacer region.
Antimycobacterial susceptibility testingStreptomycin, isoniazid, rifampicin and ethambutol susceptibility testing for Mycobaterium tuberculosis were performed according to the Method of Proportion (MOP) procedure with a rapid qualitative procedure (BACTEC™ MGIT™ 960 SIRE Kit), used with a semi-automated system (BACTEC™ MGIT™ 960 System). An isolate was determined resistant if one or more of the test population grew in the presence of the critical concentration of the drug. The testing for pyrazinamide requires some modification from the general methods because the drug is active in vitro only at lower pH values. So, pyrazinamide susceptibility testing based on the same principles but in this case a different qualitative procedure was used with a non-radiometric method (BACTEC™ MGIT™ 960 PZA Kit).
Statistical analysisData analysis was executed by IBM SPSS Statistics® for Windows version 20.0. All patient demographic and clinical features were reported using frequency and descriptive analyses. Sensitivity, specificity, positive and negative predictive measures were calculated for each and all sputum sample analysis.
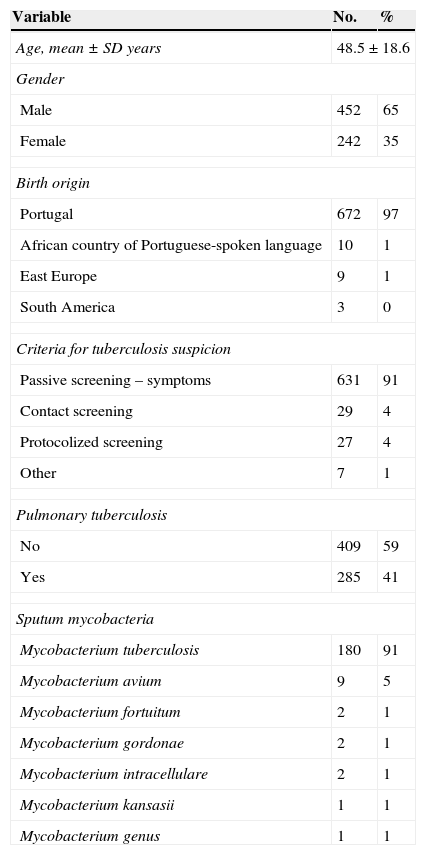
ResultsA total of 753 patients were included in the present study. Thirty-one patients were excluded as 10 of these had latent tuberculosis infection, 18 had extrapulmonary tuberculosis and three were diagnosed based on clinical grounds alone, with no microbiological or histological confirmation. Demographic and clinical characteristics of the remaining 694 patients are categorized in Table 1. Mean (range) age was 48.5±18.6 (17.1–87.4) years old. There was a non-significant predominance of male gender and a vast prevalence of Portuguese patients. A minor cluster was foreign-born, from geographical areas such as Portuguese-speaking African countries, East Europe, South America and Middle East.
Demographic and clinical characteristics of patients suspected of pulmonary tuberculosis.
| Variable | No. | % |
|---|---|---|
| Age, mean±SD years | 48.5±18.6 | |
| Gender | ||
| Male | 452 | 65 |
| Female | 242 | 35 |
| Birth origin | ||
| Portugal | 672 | 97 |
| African country of Portuguese-spoken language | 10 | 1 |
| East Europe | 9 | 1 |
| South America | 3 | 0 |
| Criteria for tuberculosis suspicion | ||
| Passive screening – symptoms | 631 | 91 |
| Contact screening | 29 | 4 |
| Protocolized screening | 27 | 4 |
| Other | 7 | 1 |
| Pulmonary tuberculosis | ||
| No | 409 | 59 |
| Yes | 285 | 41 |
| Sputum mycobacteria | ||
| Mycobacterium tuberculosis | 180 | 91 |
| Mycobacterium avium | 9 | 5 |
| Mycobacterium fortuitum | 2 | 1 |
| Mycobacterium gordonae | 2 | 1 |
| Mycobacterium intracellulare | 2 | 1 |
| Mycobacterium kansasii | 1 | 1 |
| Mycobacterium genus | 1 | 1 |
The underlying criteria for the investigation of tuberculosis among this population were mostly clinical suspicion of tuberculosis based on symptoms and/or radiological changes. A small number of patients were diagnosed while being screened for tuberculosis contacts or while submitted to screening protocols, such as those aimed at rheumatoid candidates for biological treatment.
From all 694 patients, 285 (41%) were diagnosed with active respiratory tuberculosis and 409 (59%) had non-confirmed respiratory tuberculosis. Amongst the latter group of patients, 17 (2%) had other mycobacteria isolated from sputum samples, within which the most frequent was Mycobacterium avium.
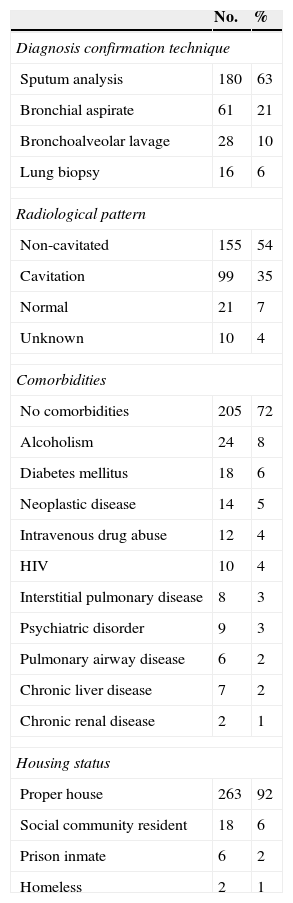
Clinical characteristics of patients diagnosed with respiratory tuberculosis are detailed in Table 2. Tuberculosis was diagnosed from sputum microbiological analysis in 180 (63%) patients. In 89 (31%) patients the disease was confirmed by the identification of Mycobacterium tuberculosis in other respiratory specimens, such as bronchial aspirate or bronchoalveolar lavage. A total of 16 (6%) symptomatic patients had tuberculosis diagnosed by histological analysis of lung biopsy samples obtained through surgical procedures and sustained by a positive IGRA test. Histological examination with hematoxylin and eosin staining revealed in all of these biopsies granulomas with central caseous necrosis ringed by epithelioid macrophages or granulomas with Langhan's giant cells along with lymphocytes, plasma cells and occasional neutrophils. Additionally, in all these cases, an acid-fast stain with Ziehl–Neelsen disclosed microorganisms as slender red rods.
Clinical characteristics of patients with pulmonary tuberculosis.
| No. | % | |
|---|---|---|
| Diagnosis confirmation technique | ||
| Sputum analysis | 180 | 63 |
| Bronchial aspirate | 61 | 21 |
| Bronchoalveolar lavage | 28 | 10 |
| Lung biopsy | 16 | 6 |
| Radiological pattern | ||
| Non-cavitated | 155 | 54 |
| Cavitation | 99 | 35 |
| Normal | 21 | 7 |
| Unknown | 10 | 4 |
| Comorbidities | ||
| No comorbidities | 205 | 72 |
| Alcoholism | 24 | 8 |
| Diabetes mellitus | 18 | 6 |
| Neoplastic disease | 14 | 5 |
| Intravenous drug abuse | 12 | 4 |
| HIV | 10 | 4 |
| Interstitial pulmonary disease | 8 | 3 |
| Psychiatric disorder | 9 | 3 |
| Pulmonary airway disease | 6 | 2 |
| Chronic liver disease | 7 | 2 |
| Chronic renal disease | 2 | 1 |
| Housing status | ||
| Proper house | 263 | 92 |
| Social community resident | 18 | 6 |
| Prison inmate | 6 | 2 |
| Homeless | 2 | 1 |
Pulmonary tuberculosis was associated with chest radiographic changes in 88% of the patients. The most frequent radiographic pattern was non-cavitated alterations (54%) but a significant number of patients exhibited cavitated lesions (34%).
At least one comorbid condition occurred in 99 patients (35%) with tuberculosis. The remaining 186 patients (65%) were otherwise healthy. Diabetes mellitus and cancer were the most frequent underlying diseases. Alcoholism and intravenous drug abuse were also not frequent; 24 and 12 patients respectively were identified.
Twenty-six patients (9%) were considered at risk for tuberculosis due to their residence status, either because they were in prison, living in community residences or were homeless.
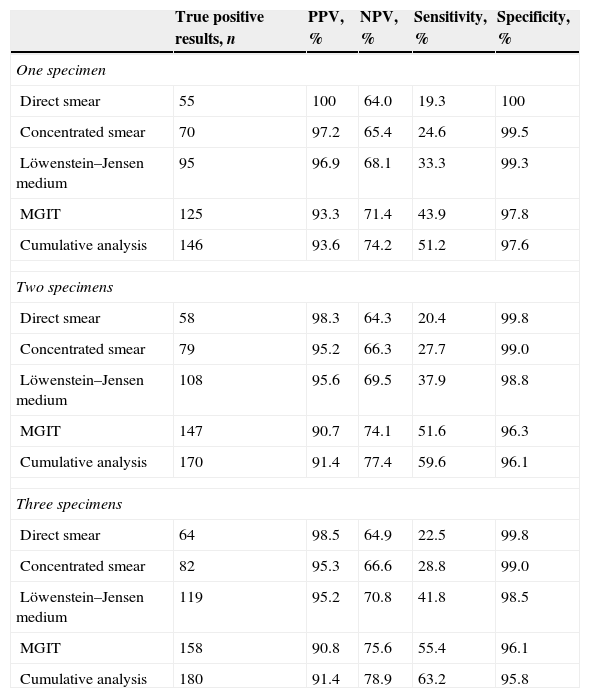
Each patient admitted to this study presented three satisfactory sputum samples for in-laboratory microbiological analysis, for a total of 2082 specimens. The diagnostic yield of all the microbiological techniques performed in the sputum samples is stated in Table 3. In terms of sputum smear microscopy, the cumulative sensitivity values for each of the three smears were 19.3%, 20.4% and 22.5% for direct smears and 24.6%, 27.7% and 28.8% for concentrated samples. Regarding mycobacterial culture, when only one specimen was tested, the sensitivities of Löwenstein–Jensen and MGIT medium incubation were 33.3% and 43.9%. When two samples were submitted the cumulative sensitivities increased to 37.9% and 51.6%, and when three were submitted sensitivities were 41.8% and 55.4%. Considering all forms of microbiological sputum analysis (direct and concentrated smear, solid and liquid medium culture), the sensitivities for the first specimen were 51.2%. The overall cumulative sensitivities values for the second and third sample were 59.6% and 63.2%.
Diagnostic yield of sputum analysis submitted to the Pulmonology Diagnostic Centre Laboratory from patients suspected of pulmonary tuberculosis.
| True positive results, n | PPV, % | NPV, % | Sensitivity, % | Specificity, % | |
|---|---|---|---|---|---|
| One specimen | |||||
| Direct smear | 55 | 100 | 64.0 | 19.3 | 100 |
| Concentrated smear | 70 | 97.2 | 65.4 | 24.6 | 99.5 |
| Löwenstein–Jensen medium | 95 | 96.9 | 68.1 | 33.3 | 99.3 |
| MGIT | 125 | 93.3 | 71.4 | 43.9 | 97.8 |
| Cumulative analysis | 146 | 93.6 | 74.2 | 51.2 | 97.6 |
| Two specimens | |||||
| Direct smear | 58 | 98.3 | 64.3 | 20.4 | 99.8 |
| Concentrated smear | 79 | 95.2 | 66.3 | 27.7 | 99.0 |
| Löwenstein–Jensen medium | 108 | 95.6 | 69.5 | 37.9 | 98.8 |
| MGIT | 147 | 90.7 | 74.1 | 51.6 | 96.3 |
| Cumulative analysis | 170 | 91.4 | 77.4 | 59.6 | 96.1 |
| Three specimens | |||||
| Direct smear | 64 | 98.5 | 64.9 | 22.5 | 99.8 |
| Concentrated smear | 82 | 95.3 | 66.6 | 28.8 | 99.0 |
| Löwenstein–Jensen medium | 119 | 95.2 | 70.8 | 41.8 | 98.5 |
| MGIT | 158 | 90.8 | 75.6 | 55.4 | 96.1 |
| Cumulative analysis | 180 | 91.4 | 78.9 | 63.2 | 95.8 |
MGIT, mycobacteria growth indicator tube; PPV, positive predicted value; NPV, negative predicted value.
The same statistical analysis was only performed for patients with pulmonary tuberculosis and chest radiography with at least one cavitated lesion. Compared to patients with other radiological patterns, patients with cavitation had higher sensitivity values for all microbiological techniques executed. The greatest sensitivity increments were observed for direct and concentrated sputum smear analysis, with an average of 13.0% and 12.4% increase in sensitivity rates, respectively. Regarding cultural analysis, the sensitivity increments were considerably lower with an average of 1.1% increase for Löwenstein–Jensen and a 5.3% increase for MGIT medium incubation. In this subset of patients, cumulative sensitivities were also higher for all three consecutive sputum sample analysis (58.6%, 63.6% and 67.7%).
There was a significant increment of sensitivity rates due to additional second and third sputum specimens. Sensitivity differences showed that, when adding a second specimen, the increment increase for direct and concentrated smear, solid and liquid medium culture was 1.1% and 3.2%, 4.6% and 7.7%, respectively. Similarly, when adding a third specimen, the sensitivity increase was 2.1% and 1.1%, 3.9% and 3.9%. The overall sensitivity difference for the two-specimen strategy was 8.4% and for the third-specimen strategy was 3.5%. For patients with cavitated lesions, cumulative sensitivity rates were 5.1% higher when adding a second, and 4.0% higher when adding a third sputum sample.
DiscussionThe microscopic staining technique is one of the earliest methods used for detecting Mycobacterium tuberculosis and it remains a standard procedure for the diagnosis of tuberculosis because it is swift, cheap and has a high positive predictive value. On the other-hand, microbiological identification of the bacilli by culture remains the gold-standard for the diagnosis of tuberculosis.
Regarding conventional microscopy, the examination of concentrated sputum offered higher diagnostic yield when compared with direct smear. Also, there was an increase in the sensitivity values when considering two or three sputum samples rather than just one. However, the greatest gain was obtained when adding a second sputum sample, while the third sputum sample offered a less substantial sensitivity increment.
Our results agree with the most consensual documented studies that show an incremental yield on more than one sputum examination19–21 and with more recent and controversial small trials claiming the relative inefficiency of the third smear and the burden it carries in undeveloped areas.9–13
The overall sensitivity of sputum smear microscopy, culture analysis and all forms of microbiological analysis together remained low and did not exceed 28.8%, 55.4% and 63.2%. As predicted, higher sensitivity rates were achieved among tuberculosis patients with pulmonary cavitation, ranging from 58.6% for the first specimen to 67.7% for the third specimen.
Low sensitivity rates may be due to pauci-bacillary disease where only a small number of Mycobacterium tuberculosis organisms are present in the respiratory specimens, and/or to technical problems. During the actual decontamination and concentration process there is tuberculosis bacilli damage that can decrease the sensitivity rates of tuberculosis detection, particularly in pauci-bacillary specimens.
One other possible explanation for a low count of bacilli disease in the tuberculosis patients observed in this study is related to the particular features of the patients observed in the PDC. In fact, the overwhelming majority was highly symptomatic and many had previously gone to their general physician or the hospital, where they had been diagnosed with pneumonia and medicated with antibiotics such as quinolones.
There are also several other technical problems that could be considered. First, the specimen collection was done at home without any kind of medical surveillance. Second, all three samples were delivered to the Laboratory on the third day and only the third sample was submitted for microbiological analysis less than 24h after being collected. Third, specific recommendations were made to each patient to preserve each sample in their domestic fridge, but again there are no guaranties that this was in fact adhered to. All of these methodological issues are directly related to the constraints of the population served by the PDC. In fact, many live far away and cannot afford to pay for three consecutive extra medical visits to provide adequate sputum samples. This strategy was adopted once patients started failing their medical appointments and active tuberculosis cases were not being diagnosed.
To overcome these real-world limitations some alternative strategies could be considered. Several studies have confirmed that the third examination is needless since two sputum smears are as effective as three smears for diagnosing tuberculosis19,21–25 and this has correspondingly been included in the current World Health Organization recommendations on sputum examination. In fact, in this study, the overall diagnostic sensitivity was incremented in 8.4% with two-specimen examination but the third-specimen added only 3.5% to sensitivity rates. Furthermore, a recent systematic review and meta-analysis showed that same-day smear microscopy is as accurate as standard microscopy stategy.26 So, a possible alternative to standard strategies could be a same-day two-specimen examination, which would diminish technical issues and reduce the number of clinical visits.
An additional methodological limitation needs to be addressed. Sputum microscopy was performed with traditional ZN microscopy but fluorescent LED microscopy could have provided higher sensitivity rates. However, and despite the fact that the WHO recommends a switch to this more sensitive procedure, this technique was not available at the PDC.
When sputum microscopy and culture are both negative, clinical diagnosis of active tuberculosis may have to rely in other conventional diagnostic methods while novel diagnostic tools and further investigation may be required to enhance the identification of Mycobacterium tuberculosis from biological specimens.
Conflicts of interestThe authors have no conflicts of interest to declare.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Institution at which work was performed: Centro de Diagnóstico Pneumológico de Coimbra, Coimbra, Portugal. Directed by: Paulo Cravo-Roxo, M.D.