Surgical lung biopsy (SLB) is still considered the gold standard procedure to obtain histology in interstitial lung diseases (ILD), the final diagnosis being decided on the basis of multidisciplinary assessment.1 The availability of a histological pattern may be very important in reaching diagnosis, predicting outcome and responding to treatment.2
There has been an increasing interest on transbronchial cryobiopsy (TCB) in the diagnosis of ILD because it presents fewer risks and complications than SLB3,4 and has a diagnostic yield superior to conventional transbronchial lung biopsy.5,6 Larger, better preserved fragments and fewer artifacts justify the greater diagnostic yield (versus conventional transbronchial lung biopsy).5,6
The aim of this work was to evaluate the diagnostic yield of TCB. All patients with suspected ILD requiring histological characterization which had been previously decided in a multidisciplinary meeting (MDM) and who underwent TCB between 05/2014 and 12/2016 were consecutively recruited into this prospective study. According to the standard of care protocol in our institution patients were required to have a pO2≥60 mmHg under oxygen delivery up to 2l/min, forced vital capacity ≥50% (predicted) and diffusing capacity of carbon monoxide ≥40% of reference. Patients with hemoglobin <8g/dL, severe pulmonary hypertension and unstable heart disease were excluded. All anticoagulant/antiplatelet therapies were discontinued before the procedure as per the BTS guidelines.7
Before the procedure, risks and possible complications were explained to each patient and informed consent was obtained.
All procedures were performed under general anesthesia and jet ventilation. The TCB was performed by a pulmonologist with experience in interventional bronchoscopy. After intubation with a rigid tracheoscope (Storz®), a videobronchoscope was advanced to the desired segment (previously identified in high resolution computed tomography) and a flexible cryoprobe (2.4mm, ERBE®) was introduced through the videobronchoscope into the bronchial segment under fluoroscopic guidance. After confirming correct positioning, a freezing time of 5s was applied. Then, the bronchoscope and cryoprobe were removed as a single unit and a bronchial blocker baloon (Olympus® B5-2c), previously placed in the segment was inflated, in order to prevent hemorrhage. The freezing agent used was nitrous oxide until June/2016 and after that carbon dioxide.
Patients were kept under observation for 2h after the procedure and had a chest X-ray. They were discharged if no complications occurred.
Statistical analyses were performed using SPSS 20.0. All variables were tested for normality using Kolmogorov–Smirnov test. Continuous variables with normal distributions were expressed as means±standard deviation. Continuous variables with non-normal distributions were summarized as medians (interquartile range). Categorical variables were expressed as numbers (percentages) and were compared using X2 test. Results were considered statistically significant when p<0.05.
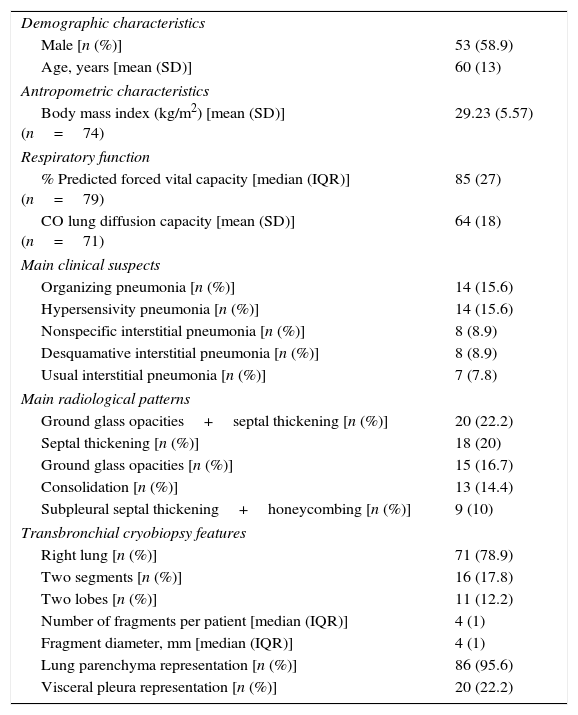
During the study period 90 patients underwent TCB. Table 1 describes their characteristics.
Patients’ characteristics.
| Demographic characteristics | |
| Male [n (%)] | 53 (58.9) |
| Age, years [mean (SD)] | 60 (13) |
| Antropometric characteristics | |
| Body mass index (kg/m2) [mean (SD)] (n=74) | 29.23 (5.57) |
| Respiratory function | |
| % Predicted forced vital capacity [median (IQR)] (n=79) | 85 (27) |
| CO lung diffusion capacity [mean (SD)] (n=71) | 64 (18) |
| Main clinical suspects | |
| Organizing pneumonia [n (%)] | 14 (15.6) |
| Hypersensivity pneumonia [n (%)] | 14 (15.6) |
| Nonspecific interstitial pneumonia [n (%)] | 8 (8.9) |
| Desquamative interstitial pneumonia [n (%)] | 8 (8.9) |
| Usual interstitial pneumonia [n (%)] | 7 (7.8) |
| Main radiological patterns | |
| Ground glass opacities+septal thickening [n (%)] | 20 (22.2) |
| Septal thickening [n (%)] | 18 (20) |
| Ground glass opacities [n (%)] | 15 (16.7) |
| Consolidation [n (%)] | 13 (14.4) |
| Subpleural septal thickening+honeycombing [n (%)] | 9 (10) |
| Transbronchial cryobiopsy features | |
| Right lung [n (%)] | 71 (78.9) |
| Two segments [n (%)] | 16 (17.8) |
| Two lobes [n (%)] | 11 (12.2) |
| Number of fragments per patient [median (IQR)] | 4 (1) |
| Fragment diameter, mm [median (IQR)] | 4 (1) |
| Lung parenchyma representation [n (%)] | 86 (95.6) |
| Visceral pleura representation [n (%)] | 20 (22.2) |
CO, carbon monoxide; IQR, interquatile range; SD, standard deviation.
A histological pattern was found by pathologists in 73.3% (n=66) of the samples (histological diagnosis). Two patients were excluded from analysis because they were lost to follow-up. After MDM and combination of clinical data, imaging, bronchoalveolar lavage and histology (TCB), a definite diagnosis was obtained when there was agreement between participants in MDM (radiologists, pathologists and pulmonologists). In 70.5% (n=62) of the cases a definite diagnosis was reached – see Table 2. Due to disagreement a definite diagnosis could not be obtained for 2 patients with a histological diagnosis. There was not a statistically significant difference between samples that provided a histologic diagnosis compared to those that did not in terms of median sample diameter.
Final diagnosis after discussion in a multidisciplinary meeting and association of clinical data, imaging, bronchoalveolar lavage and histology (TCB).
| Final diagnosis (TCB+MDM) | n=62 |
|---|---|
| Hypersensivity pneumonia | 17 |
| Organizing pneumonia | 10 |
| Smoking related ILD | |
| Desquamative interstitial pneumonia | 6 |
| Langerhans cell histiocytosis | 1 |
| Smoking related interstitial fibrosis | 1 |
| Secondary usual interstitial pneumonia | |
| Interstitial pneumonia with autoimmune features | 2 |
| Hypersensivity pneumonia | 2 |
| Drug-related | 1 |
| Connective tissue disease | 1 |
| Undefined etiology | 1 |
| Idiophatic pulmonary fibrosis | 5 |
| Familiar pulmonary fibrosis | 2 |
| Interstitial pneumonia with autoimmune features | 2 |
| Resolving pneumonia | 2 |
| Nonspecific interstitial pneumonia | 2 |
| Sarcoidosis | 1 |
| Silicosis | 1 |
| Connective tissue disease related ILD | 1 |
| Pleuroparenchymal fibroelastosis | 1 |
| Alveolar hemorrhage (drugs) | 1 |
| Vasculitis | 1 |
| Pulmonary siderosis | 1 |
TCB, transbronchial lung criobyopsy; ILD, interstitial lung disease; MDM, multidisciplinary meeting.
A definite diagnosis was not achieved for 26 patients (29.5%) with histology (TCB) and MDM. A SLB was carried out for 6 patients and the diagnosis after MDM were: 1 hypersensivity pneumonia (HP), 2 secondary usual interstitial pneumonia (1 HP and 1 of undefined etiology), 1 idiopathic pulmonary fibrosis, 1 nonspecific interstitial pneumonia and 1 silicosis. There were 6 patients with unclassifiable ILD: 1 refused SLB and 5 were not fit for surgery. For 11 patients a working diagnosis based on clinical and radiological evolution and MDM was established (4 drug-related ILD, 3 smoking-related ILD, 2 HP, 1 vasculitis, 1 sarcoidosis). Three patients remained under investigation/surveillance.
Eleven patients were submitted to two-lobe TCB. The criteria to perform two-lobe TCB were the presence of different radiologic patterns in chest computed tomography in distinct areas. The diagnostic yield (histology) of two-lobe TCB was 91% comparing to 73% one-lobe TCB (p=0.279).
Regarding complications, pneumothorax occurred in 22 patients (22.4%), 18 of which required chest drainage. Pneumothorax was associated with the presence of visceral pleura in TCB samples and there was no association with fibrotic histology and sample median diameter. There was moderate bleeding (>100ml) in 5 patients (5.6%).
TCB is a useful and safe technique in the diagnostic evaluation of ILD. When used with other data and MDM, a diagnosis is achieved in most cases, avoiding a SLB. Our diagnostic yield, 73.3% (histology) and 70.5% (TCB+MDM) is consistent with what is reported in the literature.3–6,8 The percentage of pneumothorax (22.4%) was higher than reported by some studies (7–12%)5,8 but similar to others (20–22%).3,4 The percentage of moderate bleeding (5.6%) was lower than in the majority of the studies, although the definition of bleeding severity among them is heterogeneous.3–5,8 The use of a bronchial blocker balloon may have been responsible for the low bleeding rate.
One advantage of this study is the sample size, which is higher than the majority in the literature. The low number of cases submitted to two-lobe TCB is a limitation and more studies are needed to evaluate if it has a diagnostic advantage comparing to one-lobe TCB.
In conclusion, TCB is a recognized technique in the diagnosis of ILD, but more data is needed to definitely place it in the diagnostic algorithm of ILD and its position in relation to SLB.
AuthorshipThis paper has 11 authors, because all of them had contributed to this study:
Raquel Marçôa, Rita Linhas, Sérgio Campainha and Sofia Neves participated in study conception, data acquisition, interpretation of results and drafting of the manuscript. Filomena Costa helped in the interpretation of imagiological data and Xiaogang Wen in interpretation of histological data. Dulce Apolinário and Ana Isabel Loureiro collected the data and followed up the patients referenced from other hospital. Ana Oliveira, Carla Nogueira and José Almeida contributed to data acquisition and interpretation of results.
All authors read and approved the final manuscript.
Conflicts of interestThe authors have no conflicts of interest to declare.