The Global Initiative for Chronic Obstructive Lung Disease (GOLD) report provides evidence-based guidelines for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (COPD). A major revision of the GOLD report was published in 2017 containing changes related to patient classification and pharmacologic treatment.1 The 2017 update presented a refined assessment tool that categorises patients into four groups (A–D) according to their symptoms and history of exacerbations, which were kept in the GOLD 2018 and 2019 revisions.2,3 As COPD becomes more severe, recommended treatments range from a single short- or long-acting bronchodilator to combinations of two bronchodilators and inhaled corticosteroids (ICS). An important change in the GOLD 2017 pharmacologic treatment algorithm, compared to its earlier 2016 version,4 is more specific guidance on escalation and de-escalation of ICS in groups C and D.1
Even though the GOLD report has been accepted globally, local clinical practice is often divergent from international recommendations.5,6 As GOLD is updated, there may be a need to reclassify patients and adjust treatment plans. To compare the efficacy of treatment for COPD in patients classified according to GOLD 2016 versus GOLD 2017, we retrospectively reviewed the medical records of 252 COPD patients followed at the Pulmonology Department of five hospitals in the central region of Portugal from November 2014 until November 2016. The GOLD recommendations in this period (2014–2016) were the same for patient classification criteria and pharmacological management.4,7,8 We excluded patients with a diagnosis or suspicion of asthma – COPD overlap and those with other chronic respiratory diseases such as interstitial lung disease, cystic fibrosis, and pulmonary neoplasia. We preserved patient anonymity and ensured data confidentiality throughout the study.
There were 221 men and 31 women, aged between 46.0 and 96.0 years old (mean±standard deviation was 70.0±10.4 years). Mean body mass index was 27.3±5.3kg/m2. The percentage of smokers, non-smokers, and ex-smokers was 15.1%, 27.3%, and 57.6%, respectively. All patients presented a post bronchodilation forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio <0.7. Patients presented a mean predicted FEV1 of 57.1±19.1%, with 38.5% with a FEV1<50%; nearly two thirds (65.2%) presented a grade ≥2 in the Modified Medical Research Council (mMRC) Dyspnoea Scale. The percentage of patients with ≥2 exacerbations in the previous year was 18.3%.
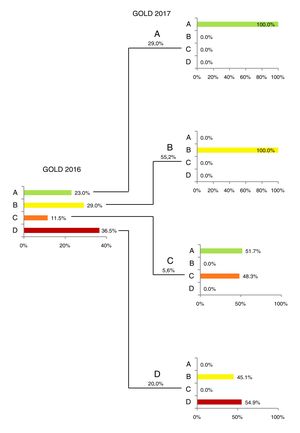
Fig. 1 presents the classification of the patients in groups ABCD according to GOLD 2016 and 2017. The largest changes were observed in groups C and D, with 51.7% of group C patients and 44.6% of group D (GOLD 2016) being reclassified according to GOLD 2017 criteria as less-severe groups A and B, respectively. A similar trend was already reported in another COPD Portuguese patient cohort.9
The most frequent comorbidity was hypertension (57.1%), followed by obesity (28.2%). Although GOLD 2018 report suggests that group B patients are likely to have comorbidities that may worsen their clinical course,2 no significant differences were found in the frequency of comorbidities among group B and the other groups.
The GOLD report offers guidance on how to tailor pharmacological treatment to a patient's disease severity. However, among patients classified according to GOLD 2016 criteria, only 9.6% received first-line therapy; 64.0% received an alternative (yet recommended) drug, and 26.4% did not receive the recommended therapy (therapy information available for 250 patients). After reclassification according to GOLD 2017 criteria (unpublished at the time), the respective percentages were 57.6%, 2.8%, and 39.6%.
Overall, the patients that did not receive the recommended therapy according to the GOLD 2016 were from the groups A (43.9%) and B (36.4%). Nineteen of the 29 inadequately treated A patients were on LABA/LAMA and 10 on ICS; all the 24 B patients were on ICS.
In the previous 12 months from data collection date, 23 (9.1%) patients stopped taking ICS, while 13 (5.2%) added ICS to their bronchodilator therapy.
It should be noted the significant increase observed in the percentage of patients treated according to the first option of GOLD 2017 versus 2016. This probably reflects one of the reasons for this major 2017 revision: the need for options adjusted to pulmonologists clinical practice.
We also highlight the fact that the patients treated outside GOLD recommendations at the time (2016), are mainly observed in groups A and B. The former due to the addition of another bronchodilator, conceivably revealing more symptomatic patients, which could be undervalued by mMRC (and would most likely be better evaluated by the COPD Assessment Test, as clearly expressed in GOLD 2019).3 In the B group, however, non-adherence was caused by the prescription of ICS in all patients. Even the possible compliance with the National Clinical Guidelines,10 last revised in 2013, does not entirely explain the divergent prescription. It would have been interesting to evaluate the level of eosinophils, a parameter which has been recently recognised by the GOLD 2019 as a biomarker for therapeutic guidance.3
Ultimately, the goal of the GOLD report is to improve the treatment of patients worldwide. Knowing and understanding our real clinical practice is one step forward to a more accurate and personalised treatment of our COPD patients.
FundingFunding for this paper was provided by Boehringer Ingelheim. Funding was used to access all necessary scientific bibliography and cover meeting expenses. Boehringer Ingelheim had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.
Conflicts of interestCR declares to have received speaking fees from AstraZeneca, Boehringer Ingelheim, Menarini, Mundipharma and Novartis.
TA has received speaking fees from Actelion, Boehringer-Ingelheim, GlaxoSmithKline, Mundipharma and Novartis, consulting fees from AstraZeneca and Boehringer-Ingelheim and a research grant from Bayer.
LF declares having received speaking fees from Boehringer Ingelheim, Medinfar, Menarini, Novartis and Tecnifar.
PF has received speaking fees from Boehringer Ingelheim, Menarini, Novartis and Mundipharma.
SS declares to have received speaking fees from AstraZeneca, Boehringer Ingelheim, Menarini, Novartis and Teva Pharma.
JCC declares no conflicts of interest.
VF declares no conflicts of interest.
ES declares no conflicts of interest.
RF declares no conflicts of interest.
We would like to acknowledge Boehringer Ingelheim for the availability and support and Prime Focus for providing statistical and writing assistance.