COPD is one of the major public health problems in people aged 40 years or above. It is currently the 4th leading cause of death in the world and projected to be the 3rd leading cause of death by 2020. COPD and cardiac comorbidities are frequently associated. They share common risk factors, pathophysiological processes, signs and symptoms, and act synergistically as negative prognostic factors. Cardiac disease includes a broad spectrum of entities with distinct pathophysiology, treatment and prognosis. From an epidemiological point of view, patients with COPD are particularly vulnerable to cardiac disease. Indeed, mortality due to cardiac disease in patients with moderate COPD is higher than mortality related to respiratory failure. Guidelines reinforce that the control of comorbidities in COPD has a clear benefit over the potential risk associated with the majority of the drugs utilized. On the other hand, the true survival benefits of aggressive treatment of cardiac disease and COPD in patients with both conditions have still not been clarified. Given their relevance in terms of prevalence and prognosis, we will focus in this paper on the management of COPD patients with ischemic coronary disease, heart failure and dysrhythmia.
Chronic diseases represent the main cause of premature death in adults worldwide.1 Chronic respiratory diseases in particular, such as asthma and chronic obstructive pulmonary disease (COPD), were directly responsible for over 3 million deaths in 2002 and affect hundreds of millions more.1,2
COPD is one of the major public health problems in people aged 40 years or more.3,4 It is currently the 4th leading cause of death in the world and projected to be the 3rd leading cause of death by 2020.2 On the other hand, according to some authors, it is the leading cause of chronic morbidity and mortality, and it is predicted to be in 7th place on the list of world disease burden in 2030.1,2
COPD and cardiac comorbidities are frequently associated. They share common risk factors, pathophysiological processes, clinical signs and symptoms, and act synergistically as negative prognostic factors.5–7 Pulmonary hypertension, right ventricular dysfunction, dysrhythmia and ischemic coronary disease are known consequences of COPD progression.8 More recently, it has been demonstrated that, in patients with COPD, left ventricular dysfunction has a negative impact on exercise tolerance, being associated with anxiety and depression, reduced carbon monoxide (CO) diffusion and higher prevalence of right ventricular dysfunction.9 Similarly, the physical activity impairment imposed by each of the above mentioned pathologies worsens the others and limits quality of life.10
Patients with COPD are particularly vulnerable to cardiac disease, with a higher incidence and prevalence (age and gender-adjusted) when compared with patients without COPD. Indeed, mortality due to cardiac disease in patients with moderate COPD is higher than mortality attributed to causes related with respiratory failure.7,11–15
Even in the general population, immediately after acute respiratory infections, a high-risk time period for cardiovascular events has been described.16 The same is true after acute exacerbations in COPD patients,17 and a higher frequency of COPD exacerbations has been associated with a higher incidence of myocardial infarction.18 In addition, cardiac biomarkers such as C-reactive Protein (CRP), fibrinogen, Brain-type Natriuretic Peptide (BNP), N-terminal proBNP (NT-proBNP), troponin, and Vascular Endothelial Growth Factor (VEGF) are higher in patients with COPD exacerbations and these are independent risk factors for mortality.19
COPD prevalence in patients with heart failure (HF) varies between 11% and 52% in the USA and between 9% and 41% in Europe,5 while the prevalence of cardiac disease in patients with COPD varies between 14% and 33%.9,20 The vulnerability to and impact of cardiac disease in patients with COPD is recognized and has been implicit in the guidelines since 2013.21 However, neither the management of cardiovascular disease (CVD) nor assessment of cardiovascular risk in patients with COPD has been studied well.22
The traditionally established and disseminated fear of beta2-agonist use in patients with cardiac disturbances and of beta-blocker (BB) use in patients with respiratory diseases is one of the reasons underlying suboptimal treatment of both conditions when they coexist, as opposed to patients with isolated COPD or CVD. Current guidelines reinforce the idea that the control of comorbidities has a clear benefit over the potential risks associated with the use of these drugs and there is no clear evidence sustaining these fears.5–7,10,23 For example, Mesquita et al conducted a research study involving COPD patients with and without impaired left ventricular ejection fraction (LVEF) and found that only half of the cohort with impaired LVEF was under targeted therapy for heart failure.9
On the other hand, the true survival benefits of aggressive treatment of cardiac disease and COPD in patients with both conditions are still not clarified.2 Cardiac disease includes a broad spectrum of entities with distinct pathophysiology, treatment and prognosis. Given their relevance in terms of prevalence and prognosis, we will focus in this paper on the management of COPD patients with ischemic coronary disease, heart failure and dysrhythmia.
Pathophysiology of COPD and Cardiovascular diseaseCardiovascular disease and COPDCOPD is a chronic pulmonary disease with an often indolent evolution and systemic repercussions.2 Its main cause is constant and prolonged exposure to cigarette smoke or other noxious gases and particles which lead to reduced air flow and pulmonary hyperinflation due to varying degrees of airway obstruction and emphysema, as well as to systemic inflammation, ultimately causing skeletal muscle dysfunction, respiratory failure, and diminished peripheral blood flow.4,24–26
In patients with COPD and CVD, the interaction of pathophysiologic processes of the respiratory, cardiac and vascular systems is complex and modulated by the action of pharmacologic agents used in the treatment of both conditions, some of which have true antagonistic effects on the autonomic nervous system. The potential side effects associated with the use of these drugs to treat COPD may translate into adverse events from a cardiovascular perspective (and vice-versa).7,27
The paradigm of the above is the use of BBs in the treatment of cardiovascular diseases and beta2-agonists in the treatment of respiratory diseases.7,28–31 The apparently opposed actions on sympathetic tone modulate cardiovascular and respiratory response and will inadvertently have implications for both systems, which is not a contra-indication for the optimized treatment of both pathologies, as discussed in this paper.
COPD effects on cardiac functionBoth diastolic and systolic dysfunction of the right and left ventricle seem to be frequent in COPD patients.8,32 Direct compromise of right ventricle (RV) is a consequence of pulmonary parenchymal destruction and hypoxic vasoconstriction resulting in increased pulmonary vascular resistance.8,32 Right ventricle dilation and hypertrophy, as a consequence of raised pulmonary pressure, can lead to septum displacement to the left ventricle, compromising left ventricular filling, stroke volume (SV) and cardiac output.33,34
Hypoxia leads to vasodilation in systemic arteries and vasoconstriction in the pulmonary vascular bed.35 However, the pulmonary hemodynamic responses to hypoxia are quite variable.35 Chronic hypoxia affects the pulmonary vasculature through both tonic vasoconstriction and vascular remodelling with miointimal hyperplasia of the pulmonary vascular bed.35
Thus, in the setting of CVD, varying degrees of hypoxia, lung hyperinflation, secondary erythrocytosis, and loss of pulmonary vascular surface area all lead to the inevitable association of CVD with varying degrees of pulmonary hypertension.35
In addition, the left ventricle may be directly affected by airflow limitation, but much less is known regarding the interaction of subclinical lung function impairment with left ventricular function.35,36 Also, these underlying mechanisms may be responsible for left ventricular failure symptoms in COPD exacerbations.35,36
Repeated and cyclic increases in ventricular wall tension are thought to be one of the mechanisms responsible for sympathetic nervous system activation in these patients, perhaps contributing to their propensity to salt and water retention, as well as the development of systemic hypertension and left ventricular hypertrophy.35,36
Some studies have reported that a moderately reduced Forced Expiratory Volume in 1 second (FEV1), an independent risk factor for CVD, is associated with an increased incidence of HF in older37 and middle-aged individuals.38,39 Impaired pulmonary function in young adulthood precedes left ventricular dysfunction later in life,40 and a linear association of decreasing FEV1/Forced Vital Capacity (FVC) ratio and increasing emphysema (hyperinflation) with left ventricular end-diastolic volume, SV and cardiac output has been shown.41,42
Pulmonary hypertension, elevated right ventricular filling pressure and raised intrathoracic pressure are also responsible for the higher incidence of atrial arrhythmias in COPD patients, probably due to a dual effect of direct raised RV pressure and pro-inflammatory environment in COPD patients.35 The greater the degree of RV dysfunction at baseline, the greater the hemodynamic significance of any added vascular load.35
Chronic inflammation in COPDBesides classical pathological models based on lung and systemic hemodynamics, there is a growing evidence on the role of the underlying local and systemic inflammatory environment. Chronic inflammation in COPD involves both innate and adaptive immunity and is most pronounced in the bronchial walls.43 This inflammatory process in COPD has a marked heterogeneity. It results in both emphysema, with parenchymal involvement, and chronic bronchitis, which predominantly affects the small airways.43 Previous studies have found persistent chronic inflammatory features in 16% of COPD patients and this was associated with a worse prognosis with a six-times higher mortality compared to patients with a pauci-inflammatory profile.44 Once established, inflammation in COPD is persistent and progresses over time, despite elimination of smoking or other environmental factors.45 Although the factors that drive inflammation in COPD have not been clearly established, autoimmunity, embedded particles from smoking and chronic bacterial infection have all been proposed to play a role.46
The inflammatory process leads to an increase in the tissue volume of the bronchial wall, characterized by infiltration of the wall by both innate (macrophages/neutrophils) and adaptive inflammatory immune cells (CD4, CD8 and B lymphocytes) with lymphoid follicles formation. Indeed, a major factor associated with lung inflammation in COPD is autoimmunity. Lee et al showed that emphysema is an autoimmune disease characterized by the presence of anti-elastin antibody and T-helper type 1 (Th1) responses, which correlate with emphysema severity.47,48
Several studies have highlighted the importance of the lung microbiome in lung disease.49–51 The most common bacteria isolated from the lungs of patients with COPD are non-typeable Haemophilus influenzae (NTHi). NTHi has been shown to induce changes in COPD in an animal model52 and new strains are also associated with COPD exacerbations.53 This agent has also been shown to activate lung T cells and cause the expression of reactive oxygen species and proteases in patients with COPD.54 The vicious cycle of inflammation-infection increases exacerbations. As evidence is growing about these mechanisms, efforts are being made to identify and define clinical and analytical biomarkers with prognostic value.
COPD and CVD biomarkersIn a prospective cohort study from the Copenhagen City Heart Study (2001-2003) and the Copenhagen General Population Study (2003-2008) population, the role of elevated levels of inflammatory biomarkers in individuals with stable COPD as predictors of exacerbations has been studied.55 The authors reported that concomitant elevated levels of C-reactive protein (CRP), fibrinogen, and leukocytes were associated with an increased risk of frequent exacerbations in individuals with stable COPD. Compared to patients with normal levels of these biomarkers, individuals with three elevated inflammatory biomarkers had an approximately 4-times higher risk of having frequent exacerbations during the first year of follow-up. It is not clear whether this might reflect an underlying bacterial colonization process, persistent latent viral infections in the airways or a highly inflammatory environment.55
The pathophysiological mechanisms and interaction between COPD and cardiac disease are complex, involving multiple biological and immune mechanisms modulating cardiac, respiratory and systemic effects.55 However, it is not possible to exactly ascertain the impact of each single mechanism in the whole process.55
From a clinical perspective, some biomarkers have been tested:
C-reactive Protein is a potential biomarker of low grade systemic inflammation and atherosclerosis in COPD. The decline in FEV1/FVC and FEV1 is correlated with an increased high-sensitivity CRP and the risk of ischemic heart disease is higher in patients with both moderate to severe obstruction and higher CRP levels.56,57
Fibrinogen is an acute phase protein that is has been described as a marker of COPD activity. High levels of this marker are a predictor of severity and risk of COPD exacerbations.58,59
Brain-type Natriuretic Peptide (BNP) and N-terminal proBNP (NT-proBNP) are early and sensitive biomarkers for the diagnosis of HF associated with decreased left ventricle ejection fraction, left ventricular hypertrophy, increased left ventricle filling pressure, acute myocardial infarction and ischemia.56,60,61 They are also higher in patients with pulmonary disorders, right ventricular dysfunction, pulmonary hypertension and cor pulmonale. In COPD patients, the increase in BNP and NT-proBNP is proportional to the severity of right ventricular dysfunction.56,60,61 BNP is also high in patients with stable COPD and no pulmonary hypertension. In addition there is a strong correlation between BNP levels and left ventricular ejection fraction and systolic pressure of the pulmonary artery.56,60,61 Finally, BNP levels have also been associated to mortality risk in COPD patients.56,60,61
Troponin is elevated in 18-27% of patients hospitalized due to a COPD exacerbation and is an independent predictor of mortality both during the exacerbation and in the long term.12,62–66
VEGF (Vascular Endothelial Growth Factor) is an important biomarker of prognosis in CVD. VEGF regulates angiogenesis, promoting the migration and proliferation of endothelial cells, increasing vascular permeability and modulating thrombogenicity. Patients with COPD exacerbations have higher levels of circulating VEGF.57
Surfactant protein D is synthesized and secreted by bronchial and alveolar epithelial cells and can be detected in human plasma. It has a major role in immune and inflammatory regulation in the lung and is also expressed in coronary arteries, having an anti-inflammatory role.57
COPD and Concomitant CVD assessment methodsPatients with COPD and cardiovascular comorbidity should have respiratory function, cardiac function, and systemic inflammatory status assessments. These assessments should be conducted differently depending on whether a patient has stable COPD or an acute exacerbation.
- A.
For patients with stable COPD, a clinical and functional assessment should include:
- •
Complete clinical history and physical examination, including the recommended dyspnea and quality of life assessment questionnaires modified Medical Research Council (mMRC) and COPD Assessment Test (CAT).
- •
Laboratory assessment including complete blood count, arterial blood gas analysis, CRP, NT-proBNP and/or BNP.
- •
Spirometry, static lung volumes and carbon monoxide diffusion capacity (DLCO).
- •
Chest X-ray.
- •
12-lead electrocardiogram and further assessment with an echocardiogram, if needed.
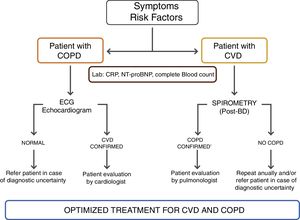
For patients with known concomitant coronary artery disease or cardiac disease, a specific assessment should be done according to the current guidelines, as proposed by the authors – Figure 1. A six minute walking test and cardiopulmonary exercise testing are recommended if there is no specific contra-indication.
- B.
For patients with cardiac disease and signs/symptoms or risk factors for COPD, COPD should be actively investigated, so, in addition to the clinical assessment specific for cardiac disease, spirometry, lung volumes and DLCO measurements should also be performed. Additional assessments should be carried out according to the results obtained from the respiratory and cardiac specific functional assessments.
For both patients mentioned in A and B sections, who frequently undergo thoracic CT scan, it is important to keep in mind that patients undergoing evaluation for cardiac disease, coronary angio-CT may show radiologic signs of COPD, and COPD patients undergoing thoracic CT may show coronary calcification or cardiomegaly suggesting underlying cardiac disease.67
Due to the multifactorial, clinical interactions and a broad spectrum of signs and symptoms, there are no specific guidelines for when and how to perform these functional cardio-respiratory assessments. There are broad rules that should be tailored to each individual patient according to clinical expertise, and the authors propose a specific assessment algorithm for CVD in stable COPD patients – Figure 1.
- C.
For patients with COPD experiencing an acute exacerbation:
- •
Complete clinical history and physical examination.
- •
Laboratory assessment including complete blood count, leukocyte count, platelets, CRP, arterial blood gas analysis, fibrinogen, NT-proBNP or BNP and troponin I.
- •
Chest X-ray.
- •
12-lead electrocardiogram.
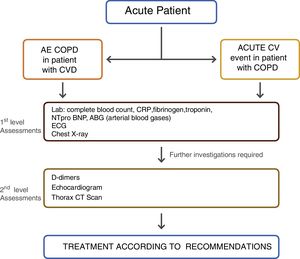
The purpose is to establish the main pathophysiologic mechanisms underlying the acute exacerbation when both pathologies co-exist: decompensated cardiac disease, COPD exacerbation, underlying infection or another concomitant diagnosis. Therapeutic approach should be driven according to the diagnosis associated with the acute clinical decompensation. The treatment of these patients can be quite challenging, especially in the acute setting. Indeed, although the reason for decompensation can be primarily cardiovascular or respiratory in nature, the authors propose that both conditions have to be simultaneously addressed, because of the constant two-way interaction of dysfunctional mechanisms – Figure 2.
COPD and CVD Pharmacological TreatmentThe presence of comorbidities should not modify COPD treatment and comorbidities should be treated per usual standards regardless of the presence of COPD.2
Cardiovascular drugs in COPD patientsCampo et al conducted a review on the safety and efficacy of cardiovascular and respiratory drugs in COPD patients with concomitant CVD. Antiplatelet agents, anticoagulants, angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), and BBs are the most commonly prescribed drugs in patients with CVD.68
Antiplatelet agents and anticoagulantsIt is reasonable to think that it might all be useful in patients with both COPD and CVD since thrombocytosis is associated with increased mortality in COPD. COPD patients submitted to percutaneous coronary interventions have higher platelet reactivity, and antiplatelet agents and anti-aggregation therapy may act as beneficial in pulmonary hypertension and in COPD patients at risk of atrial fibrillation.69–71
According to the available evidence, antiplatelet therapy has been associated with a reduced 1-year mortality (OR 0.63; 95% CI 0.47–0.85) in patients with hospital admission due to acute exacerbation of underlying COPD.68 Similarly, Ekstrom et al showed that antiplatelet agents were associated with a significant reduction of mortality in COPD patients (HR 0.86; 95% CI 0.75–0.99).30 However, these data are based on post-hoc analysis and no RCTs were conducted specifically on this issue.
The benefit of anticoagulant drugs relies on the concomitant CVD independently of the COPD status.68,72 However, COPD seems to be a risk factor for bleeding complications,73,74 namely in patients with atrial fibrillation on anticoagulant therapy.75,76
ACEi and ARBsSmall clinical trials and observational studies have suggested an interaction between ACEi and FEV1 decline in smokers whilst others suggest a benefit in pulmonary hypertension in patients treated with ARBs.68,77,78 However, there is not sufficient evidence to define a specific indication of ACEi or ARBs in COPD patients other than for the CVD.68,77,78
BBsA much larger body of evidence and literature is available about indication and prescription of BBs. Bronchoconstriction from β-blocker use is due to antagonism of pre-junctional and post-junctional β-2 receptors, which uncovers cholinergic tone resulting in airway constriction.6 Given this rationale, β-1 selective antagonists potentially exhibit a small degree of dose-related β-2 receptor blockade. A meta-analysis including studies until 2011 concluded that BBs have a positive effect in COPD patients with ischemic heart disease, and cardio-selective BBs produce no change in FEV1 or respiratory symptoms, nor do they affect the FEV1 treatment response to long acting β-2 agonists (LABA), and it showed a pooled relative risk reduction in mortality for COPD patients receiving BB (RR 0.69, 95% CI 0.62–0.78).68,79 A possible explanation for this positive interaction is the protector effect of cardio-selective BB against the potential chronotropic, inotropic and pro-arrhythmic effects of LABA.
Despite the safety of BBs in COPD patients, the treatment with BBs or ACEi/ARB was found to be significantly lower in COPD patients with concomitant HF than in patients with HF alone.28–30 The use of BBs has also been reported to be 41% to 58% lower than ACEi/ARB in patients treated with ICS/LABA/LAMA or SAMA/SABA.6,68 A very recent review proposes that potential ways of dealing this dilemma of whether or not to use BBs in COPD patients include the development of highly β1-selective β-blockers or the use of non-β-blocking heart rate reducing agents, such as ivabradine, if these are proven to be beneficial in randomised controlled trials.80
The authors conclude that the above mentioned drug classes for COPD can be safely used if concomitant CVD is an indication.
The use of cardioselective BBs in COPD patients seems to be safe and may be suggested if indicated for the CVDper se. Cardio-selective BBs, if indicated, may be recommended to COPD patients, regardless of pulmonary comorbidity.6,31,68,81
Respiratory drugs in CVD patientsAny bronchodilator is potentially pro-arrhythmic. Although some studies report an incidence of tachydysrythmias in LAMA treated COPD patients,68 the UPLIFT82 and TIOSPIR83 trials did not show an increased incidence of major cardiac events. The cardiovascular safety of LABA, LAMA, ICS/LABA, or LABA/LAMA therapy is well established.7,10,84–88 Specifically, a fixed once-daily combination of indacaterol/glycopyrronium has shown cardiovascular safety in COPD patients, with or without comorbid CVD.23,85,89–91 Moreover, a very recent RCT has shown that dual bronchodilation with indacaterol/glycopyrronium significantly improved cardiac function in patients with COPD and lung hyperinflation.92
Regarding tachydysrythmias 2 there is no evidence that the COPD treatment approach should be changed due to the diagnosis of atrial fibrillation or another CVD.
Specific considerations regarding short-acting beta agonists (SABAs)Due to their β2-adrenergic action, SABAs may precipitate atrial fibrillation and hamper the control of ventricular response, and have the potential to induce sinus tachycardia at rest and dysrhytmias in susceptible patients.93 However, if clinically indicated, they should be used as rescue medication.
Specific considerations regarding LABAsAlthough the cardiovascular safety of LABAs in COPD patients remains a controversial issue, a recent meta-analysis of 24 RCTs concluded that it is safe to use LABAs in COPD patients with CV comorbidities.87
Specific considerations regarding LAMAsLAMAs are safe within a wide range of doses and clinical contexts. Some concerns have been raised regarding tiotropium administration via the Respimat® device, but the TIOSPIR study showed it to be as safe as tiotropium administred via the Handihaler®. Glycopyrronium also has a favorable safety profile with no increase in CV risk. The safety profile is very similar amongst the different LAMAs.2,79,88,94,95
Specific considerations regarding ICSICS are not used as monotherapy in COPD patients. ICS safety data are less robust than safety data on bronchodilators. There is no unequivocal evidence regarding safety of ICS used as combination therapy on COPD patients with comorbid CVD.7,79,88
Specific considerations regarding methylxanthinesMethylxanthines have a narrow therapeutic window and their toxicity is well established and dose-dependent. With regard to CVD, their pro-arrhythmic effect should be highlighted. There is also a potential pharmacokinetic interaction with warfarin and digoxin.2 Theophylline has been suggested to have an anti-inflammatory effect in patients with COPD.96
In conclusion, ICS, LABAs, LAMAs and associations can be considered safe in COPD patients with concomitant cardiac disease.
Prognostic ImplicationsCOPD patients with concomitant CVD have a worse prognosis than simply the sum of the prognosis of each disease. However, it is difficult to establish a hazard ratio or risk ratio for a specific patient or group of patients, due to the complex etiologic and pathophysiologic interaction network that underlies both diseases.20,23 Much of the literature relies on epidemiology data and management of cardiovascular comorbidities in COPD. Much less is described regarding the prognostic implications of different cardiovascular comorbidities in COPD patients and even less in subgroups of COPD patients, both according to GOLD stages and age.
Summarizing the literature, the risk of CVD in COPD is two to three-fold greater than the risk associated with smoking.97 COPD is also independently associated with meaningful cardiovascular events.98 FEV1 is an independent risk factor for cardiovascular disease regardless of age, gender, tobacco addiction, cholesterol, and education level/social class.98 Reduced pulmonary function in COPD is associated with increased incidence of all-cause mortality, cardiovascular-related mortality, myocardial infarction and arrhythmias.99 In the TORCH trial, cardiovascular deaths occurred in 26% of patients,14 and in the UPLIFT trial in 18.8% of patients.100 Both trials included patients with moderate to severe COPD. Cardiovascular death remains the most common cause of mortality in COPD patients.101
ConclusionsIn conclusion, the identification and proper treatment of cardiac comorbidity is a very important measure in the management of COPD, as prognosis in COPD is greatly affected by the presence of CVD. Due to pathophysiological and inflammatory interactions between COPD and cardiovascular disease, the presence of cardiac comorbidities should be actively investigated in all COPD patients, both in stable conditions and in the context of exacerbations.
Role of Funding SourceFunding for this paper was provided by Novartis Portugal. Funding was used to access all necessary scientific bibliography and cover meeting expenses. Novartis Portugal had no role in the collection, analysis and interpretation of data, in the writing of the paper and in the decision to submit the paper for publication.
Conflict of InterestSA declares having received speaking fees from Novartis and participating on scientific advisory boards sponsored by Novartis. BC declares having received speaking fees from Novartis and participating on scientific advisory boards sponsored by Novartis. EF declares having received speaking fees from Novartis, Boehringer Ingelheim, GSK, Menarini, AstraZeneca, Teva, Medinfar, Tecnifar and Mundipharma and participating on scientific advisory boards sponsored by Boehringer Ingelheim. JPBT declares having received speaking fees from Novartis. VA declares having received speaking fees from Novartis, Medinfar and GSK. JC declares having given presentations at symposia and/or served on scientific advisory boards sponsored by AstraZeneca, Boehringer Ingelheim, GSK, Mundipharma and Novartis.