The role of nocturnal non invasive ventilation (NIV) to prolong tracheostomy-free survival, is still controversial in amyotrophic lateral sclerosis (ALS) patients and the best timing to initiate NIV is unclear.
ObjectiveAs NIV acceptance and adherence can be influenced by many factors, we aimed to compare immediate acceptance and short-term NIV adherence between NIV initiated very early and NIV initiated later.
MethodsThis is a post hoc analysis of our previous cohort retrospective study of 88 ALS patients: 53 under later NIV (late group – LG) (forced vital capacity [FVC]<80% pred. at NIV prescription) and 35 under very early NIV (very early group – VEG) (FVC>80%). We compared hours of NIV use as immediate acceptance of NIV (use ≥4h/night) and dherence at 4 months post-initiation (defined as use ≥4h/night or 120h/month).
ResultsNo differences were found between VEG and LG in use of NIV (>5h/night in both groups), immediate acceptance (85.7% vs. 85.0%, p=0.927) and short-term adherence (81.3% vs. 87.2%, p=0.469); 39.7% of patients increased their NIV use (35% by >60min/night). A decline in adherence was observed in 12.5% of patients irrespective of group affiliation.
ConclusionsIn ALS patients, initiation of very early NIV does not reduce its immediate acceptance or the short-term adherence. However, at least 1 in 10 patients may be at risk of reducing their adherence irrespective of early or late NIV prescription. As still under debate and not conclusive, further literature on early NIV benefit is welcomed.
The role of nocturnal non invasive ventilation (NIV) or continuous non invasive ventilatory support (CNVS) to prolong tracheostomy-free survival is still controversial in amyotrophic lateral sclerosis (ALS) patients.1,2 Also the synergistic effect of NIV initiation and use of cough assisted devices (CAD) to postpone death with or without a CNVS dependence remains logical, but still unclear.1,2 Both Jacobs et al.3 and the EFNS Guidelines2 stress the urgent need to evaluate the impact of early NIV initiation on survival in ALS patients.
If the timing of NIV initiation may also influence adherence4 is indeed a relevant question: over the years, the literature has not produced sufficient or robust enough evidence. This fact is mainly due to the empirically driven criteria, essentially based on a percentage of predicted FVC, instead of combining it with the maximal inspiratory pressure, which always comes first.2,4
Acceptance of and adherence to NIV can be influenced by site of disease onset, NIV settings and comfort, asynchrony presence, aggressive disease, ALS phenotype, and on top of these psychological defense to clinical status.2–7 Many patients stop NIV with time since they feel they have not benefited from it while others reconsider NIV use when it relieves their symptoms. Previous studied have demonstrated7 that outpatient NIV initiation in ALS is not inferior to inpatient NIV initiation in terms of patients’ acceptance and adherence. Recently, it has also been shown8 that the mortality risk for ALS patients who are initiated very early on NIV may be lower than for patients initiated at a later stage.
For the present study, the authors compared immediate NIV acceptance and adherence at 4 months between very early group (VEG) and later group (LG). The study was approved by the Ethical Committee (CEC 706, 18 April 2011; 24 January 2017).
MethodsThis is a post hoc analysis on previous cohort studies database8,9 derived from medical charts of 109 ALS patients admitted in the period 2008–2013 to two (ICS Maugeri Lumezzane (Brescia) and Don Gnocchi Foundation IRCCS Onlus (Milano)) out of the three Italian facilities which participated to the previous study.8
Eighty eight patients with ALS diagnosis followed for NIV indication in which the NIV adherence report (hours of nocturnal NIV) was available, were re-analyzed. The patients’ immediate acceptance of NIV was defined as NIV use ≥4h/night within 10 days of initiation to provide a definitive prescription. The patients’ short term adherence to NIV was defined as NIV use ≥4h/night or 120h/month within 4 months following initiation.9 In addition the number of patients whose NIV adherence had declined, or was unchanged, or had increased (both night-time and daytime) with respect to T0, was evaluated.
The “real life” decisions to prescribe NIV were sometimes according to guideline recommendations,2 sometimes only on the physician's own clinical judgment with initial Pimax or Pemax % impairment.8 NIV pressure settings were adjusted so that the patient felt comfortable and could actually tolerate NIV; the hours prescribed per day were the maximum the patient could tolerate.8,9
In the above-mentioned study9 and in the present study, patients were arbitrarily divided into two groups9 according to the sitting FVC% pred. at the time of NIV prescription: LG with FVC<80% and VEG with FVC≥80%. NIV initiation was performed with pressure-support ventilators in spontaneous/timed mode with a preset tidal volume (5ml/Kg) and a fixed back-up respiratory rate (12breaths/min). NIV trials included choice of the best fitting mask (nasal/oronasal), setting of pressures to maximal patient comfort, and advice to use nocturnal NIV as much as possible. If necessary, the ventilator setting could be further adjusted to optimize nocturnal oximetry/polysomnography, partial pressure of CO2 (paCO2) normalization and NIV adherence (at least 4h/night or 120h/month of NIV use).8,9 During the 4-month of follow-up, patients were able to contact at any time (both by phone or directly with a face to face visit) the multidisciplinary care team (nursing, respiratory physical therapy, neurological, respiratory and psychological second opinion) to receive support for any reason related to NIV and their clinical condition.
Unpaired t-test for continuous variables and proportion tests for categorical and binary variables were used. Due to the retrospective nature of the study, no specific power calculation was made.
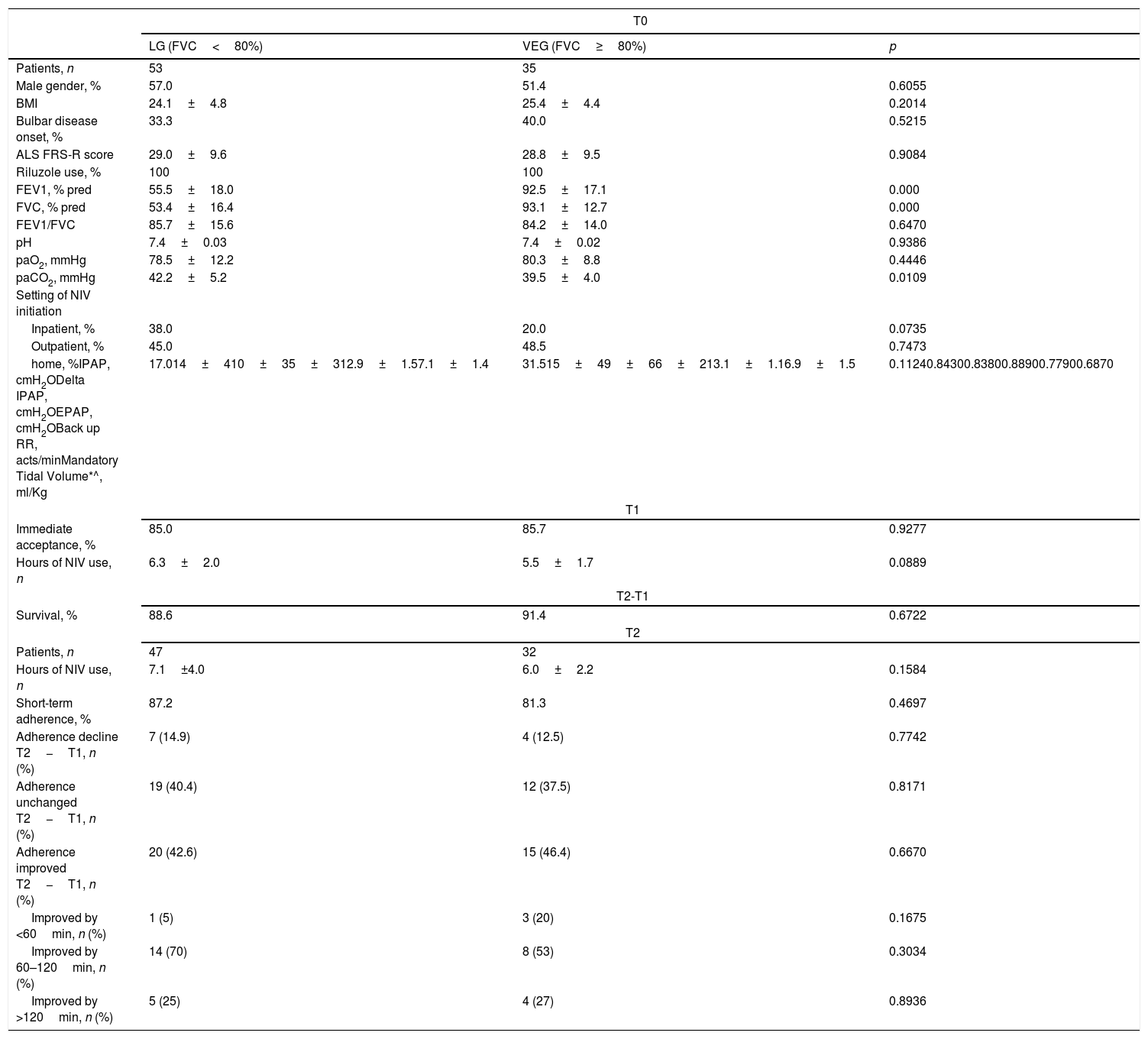
ResultsA total of 88 patients were studied, 53 in LG and 35 in VEG. No differences were found in the rate of immediate acceptance (Table 1), with more than 5h of NIV use per night in both groups as well. The main reasons for NIV intolerance and poor adherence were mask troubles/claustrophobia (22%), inability to move the mask independently (18%), inadequacy of the caregiver (20%), depressive symptoms with disease and NIV refusal (40%), irrespective of the two groups.
Anthropometric, functional, clinical characteristics and outcomes of ALS patients according to late vs. early initiation of NIV.
| T0 | |||
|---|---|---|---|
| LG (FVC<80%) | VEG (FVC≥80%) | p | |
| Patients, n | 53 | 35 | |
| Male gender, % | 57.0 | 51.4 | 0.6055 |
| BMI | 24.1±4.8 | 25.4±4.4 | 0.2014 |
| Bulbar disease onset, % | 33.3 | 40.0 | 0.5215 |
| ALS FRS-R score | 29.0±9.6 | 28.8±9.5 | 0.9084 |
| Riluzole use, % | 100 | 100 | |
| FEV1, % pred | 55.5±18.0 | 92.5±17.1 | 0.000 |
| FVC, % pred | 53.4±16.4 | 93.1±12.7 | 0.000 |
| FEV1/FVC | 85.7±15.6 | 84.2±14.0 | 0.6470 |
| pH | 7.4±0.03 | 7.4±0.02 | 0.9386 |
| paO2, mmHg | 78.5±12.2 | 80.3±8.8 | 0.4446 |
| paCO2, mmHg | 42.2±5.2 | 39.5±4.0 | 0.0109 |
| Setting of NIV initiation | |||
| Inpatient, % | 38.0 | 20.0 | 0.0735 |
| Outpatient, % | 45.0 | 48.5 | 0.7473 |
| home, %IPAP, cmH2ODelta IPAP, cmH2OEPAP, cmH2OBack up RR, acts/minMandatory Tidal Volume*^, ml/Kg | 17.014±410±35±312.9±1.57.1±1.4 | 31.515±49±66±213.1±1.16.9±1.5 | 0.11240.84300.83800.88900.77900.6870 |
| T1 | |||
| Immediate acceptance, % | 85.0 | 85.7 | 0.9277 |
| Hours of NIV use, n | 6.3±2.0 | 5.5±1.7 | 0.0889 |
| T2-T1 | |||
| Survival, % | 88.6 | 91.4 | 0.6722 |
| T2 | |||
| Patients, n | 47 | 32 | |
| Hours of NIV use, n | 7.1±4.0 | 6.0±2.2 | 0.1584 |
| Short-term adherence, % | 87.2 | 81.3 | 0.4697 |
| Adherence decline T2−T1, n (%) | 7 (14.9) | 4 (12.5) | 0.7742 |
| Adherence unchanged T2−T1, n (%) | 19 (40.4) | 12 (37.5) | 0.8171 |
| Adherence improved T2−T1, n (%) | 20 (42.6) | 15 (46.4) | 0.6670 |
| Improved by <60min, n (%) | 1 (5) | 3 (20) | 0.1675 |
| Improved by 60–120min, n (%) | 14 (70) | 8 (53) | 0.3034 |
| Improved by >120min, n (%) | 5 (25) | 4 (27) | 0.8936 |
ALS=amyotrophic lateral sclerosis; VEG=very early group; LG=late group; NIV=noninvasive ventilation; FVC=forced vital capacity; BMI=body mass index; ALS FRS-R=ALS Functional Rating Scale Revised; FEV1=forced expiratory volume in the 1st second; paO2=partial arterial oxygen pressure; paCO2=partial arterial pressure of carbon dioxide; IPAP=inspiratory positive airway pressure,*=For AVAPS; EPAP expiratory positive airway pressure; RR=respiratory rate; ^=for volumetric; T0: baseline; T1: 30 days from NIV initiation; T2: 4 months from NIV initiation.
In the surviving patients (n=79), the rate of short-term adherence was similar (Table 1). In both groups, 44.3% of patients increased their NIV use (39% of these improvers used NIV more than 60min/night): VEG group increased NIV use in 48.4%, while LG in 43.5% (p=0.6670). In contrast, in 13.9% patients there was a decline in NIV adherence (in 12.9% of VEG patients and in 15.2% of LG patients, p=0.7742). No differences were found in survival at the 4-month follow-up. In the VEG, bulbar involvement did not influence negatively NIV adherence at 3 months (81.8% in bulbar and 77.3% in non-bulbar subgroups p=0.763) while in the LG, bulbar patients adhered better than non-bulbar ones (94.7% vs. 69.7% p=0.033).
DiscussionTo evaluate the possible impact of early NIV initiation on survival in ALS patients we have retrospectively9 analyzed when NIV was prescribed as a function of FVC was greater than or less than 80%. This may be considered arbitrary. At the same time discussion of “survival” without indicating causes of death is often not relevant. The combination of NIV/CNVS and CAD,1 probably greatly extends survival more than daily NIV or when it actually started.
Anyway, for the present study, we compared the immediate (within 10 days) NIV acceptance and short term adherence at 4 months between patients adapted very early (FVC>80%) or later (FVC<80%). In real life experience, patients’ symptoms are probably the most important factor for NIV tolerance, whether bulbar or not. There may be speculation about whether “early” NIV timing could theoretically be refused by patients because they are not yet severely affected (as bulbar ones are) by disease worsening (and hence less compliant) while those coming to treatment later present a higher incidence of complicating factors. Starting NIV progressively when respiratory function is not yet severely compromised may make it less uncomfortable for patients to adhere to NIV. On the other hand, we have recently shown9 that very early NIV initiation can postpone death in ALS patients (in particular, in the non-bulbar subgroup). In the present study the majority of patients (85%) became completely confident with the NIV use within 4 months irrespective of early or late NIV initiation. An important practical warning coming from our data is that, after only 4 months after starting NIV a subgroup of patients showed a decline in adherence irrespective of whether initiation was early or late, probably because some patients did not accept their condition rather than the ventilator per se.
As limitations, we can subtitle that (1) longer observation (>4 months) would have been useful to figure out the differences between the two groups because a certain proportion of patients reduced adherence irrespective of group affiliation, (2) no data have been presented on ventilator/patient interaction, asynchrony data, time course of disease aggression or psychological defense mechanism as possible reasons for reduction in immediate or short term NIV acceptance.
In conclusion, very early NIV initiation in ALS does not appear to reduce its immediate acceptance by patients, nor its short-term adherence, compared to later initiation of NIV. However, at least 1 in 10 patients may be at risk of showing a decline in adherence, irrespective of whether the NIV initiation is early or late. As this subject is still under debate and not conclusive, further literature on early NIV benefit is welcomed.
FundingThis work was supported by the “Ricerca Corrente” Funding scheme of the Ministry of Health, Italy.
Conflicts of interestThe authors have no conflicts of interest to declare.