Pulmonary rehabilitation (PR) is a cornerstone intervention for the management of patients with stable chronic obstructive pulmonary disease (COPD). However, its role during acute exacerbations (AECOPD) is controversial since most studies have been conducted in hospitalised patients, when more than 80% of AECOPD are managed on an outpatient basis. This quasi-experimental pilot study assessed the effects of a community-based PR programme during mild-to-moderate AECOPD.
MethodsOutpatients were recruited from hospitals and allocated to experimental (EG) or control (CG) groups. EG received standard medication plus 3-weeks of PR. The CG received standard medication. Dyspnoea (mMRC), quadriceps muscle strength (QMS), functionality (5-repetition sit-to-stand test) and impact of the disease (COPD assessment test (CAT)) were assessed within 48h of the AECOPD onset and after PR. Symptoms of dyspnoea and fatigue (mBorg), heart and respiratory (RR) rates and peripheral oxygen saturation (SpO2) were assessed at rest and monitored in all PR sessions. Need for hospitalisation was monitored during the 3-weeks.
ResultsTwelve patients (69±7 years, FEV1 52±27 pp) in the EG and eleven in the CG (66±9 years, FEV1 55±22 pp) were enrolled. The EG presented significant improvements on QMS (Pre 21.0 vs. Post 25.0, p=0.012), CAT (Pre 23.0 vs. Post 14.5, p=0.008), symptoms of dyspnoea at rest (Pre 3.0 vs. Post 1.0, p=0.008), SpO2 (Pre 94.0 vs. Post 96.0, p=0.031) and RR (Pre 24.0 vs. Post 20.5, p=0.004). No significant improvements were found in the CG.
ConclusionAdding PR to the management of mild-to-moderate AECOPD seems to result in improvements on parameters usually associated with an increased risk of re-exacerbation and poor prognosis. Randomised studies with larger samples are needed to confirm these results.
The trajectory of chronic obstructive pulmonary disease (COPD) is frequently punctuated by exacerbations, defined as episodes of acute worsening of respiratory symptoms that result in additional therapy.1,2 Acute exacerbations of COPD (AECOPD) account for more than 70% of all COPD-related costs3 and are mainly responsible for patients’ clinical deterioration.1 It is known that AECOPD result in significant decline in muscle strength, functional capacity and lung function, which further impairs patients’ health-related quality of life (HRQoL) and increases their susceptibility to more exacerbations, hospitalisations and death.1,2,4,5 Therefore, treatment goals for patients with AECOPD are to minimise the negative impact of these events and prevent their recurrence.1
Pulmonary rehabilitation (PR) is a well-established intervention for the management of patients with stable COPD.1,6 It has been shown to: (i) improve exercise capacity, functional capacity, muscle strength and HRQoL; (ii) reduce symptoms, hospitalisations and unscheduled healthcare visits; and (iii) enhance self-management and self-efficacy.6 Given these benefits it would seem reasonable to consider PR as a management strategy for AECOPD.7 However, studies assessing the role of PR during AECOPD have shown controversial results;1,8 this is probably related to the settings in which the PR programmes have been conducted and the disease severity of patients included. Most studies have been conducted in hospitalised patients,3,9 who present more severe exacerbations and/or more severe underlying disease than those managed on an outpatient setting.1 Although it is recognised that more than 80% of all AECOPD are managed on an outpatient basis,1 only a few studies have conducted PR programmes on an outpatient setting.10–13 Additionally, those studies did not start their intervention at the onset of the AECOPD but shortly after the AECOPD,10,11 included patients with AECOPD who have previously been hospitalised,12,13 and have found significant results in different outcomes (e.g., exercise capacity,10,12 HRQoL,10–12 and re-exacerbations13). Thus, the potential role of PR programmes during AECOPD in patients managed on an outpatient basis is not yet clarified. Moreover, outpatient basis includes community-based PR (i.e., PR programmes nearby patients’ homes), which may be a promising approach to overcoming the shortcomings of poor access and transport to PR, especially in patients with AECOPD, but has hardly been investigated.14,15
Therefore, this pilot study aimed to explore the feasibility of conducting a community-based PR programme during AECOPD and assess its effects on patients’ symptoms, muscle strength, functionality, impact of the disease, peripheral oxygen saturation and hospitalizations.
MethodsEthicsApproval for this study was obtained from the ethics committees of the Administração Regional de Saúde do Centro, I.P. (3NOV’2016:64/2016), Centro Hospitalar do Baixo Vouga (22MAR’2017:777638), Hospital Pedro Hispano (17FEB’2017:10/CE/JAS) and Hospital Distrital da Figueira da Foz (18JUL’2017) and from the National Data Protection Committee (8828/2016). Written informed consents were obtained from all participants before any data collection.
Study design and participantsA quasi-experimental study was conducted in outpatients with AECOPD recruited from three main hospitals between November 2016 and December 2017. Inclusion criteria were diagnosis of AECOPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria.1 Exclusion criteria were: (i) hospitalisation; (ii) presence of severe co-existing cardiac, respiratory, neurological, musculoskeletal, or signs of psychiatric impairments; (iii) current neoplasia or immunological disease and (v) any therapeutic intervention in addition to standard of care. Eligible patients were identified by pulmonologists and contacted by the researchers, who explained the purpose of the study and asked about their willingness to participate. An appointment with the researchers was scheduled within 48h of the diagnosis of AECOPD with those interested in participating. Due to the exploratory nature of this study and the controversy around the efficacy and safety of conducting PR during AECOPD,2 we felt that randomising patients was not suitable.16 Like other studies,17–19 patients were given the choice of treatment. Participants who accepted enrolment in the PR programme were assigned to the experimental group (EG), the remaining participants, who accepted participation in the study but did not want to be enrolled in the PR programme, composed the control group (CG).
Sample sizeAccording to the recommendations for adequate sample sizes to conduct pilot studies, twelve participants in each group would be needed to conduct this study.20 However, as it is known that dropout rates in respiratory interventions are around 30–35%,21 sixteen patients in each group were aimed to be recruited.
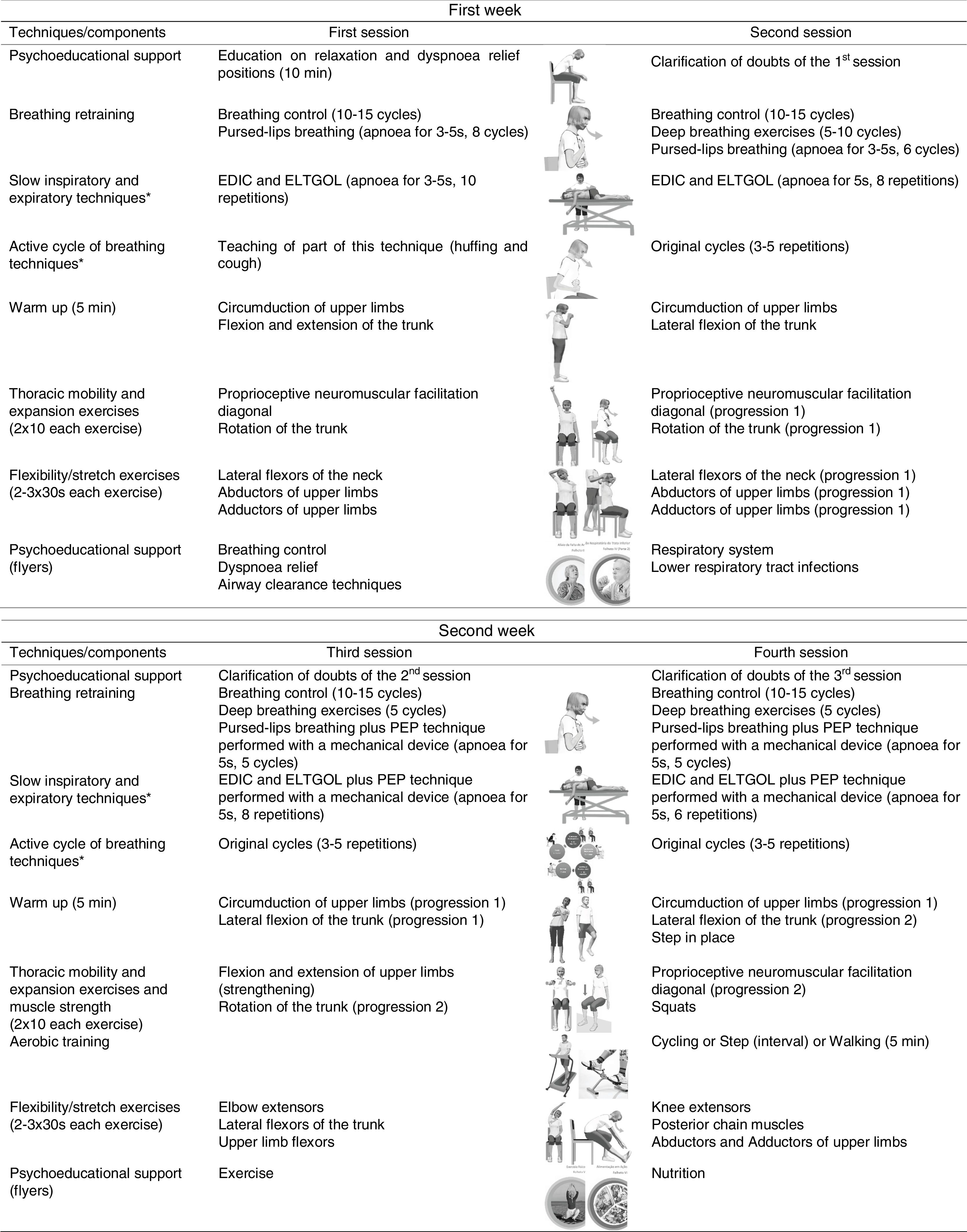
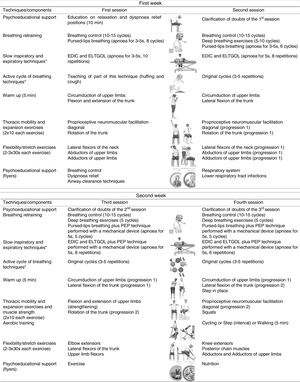
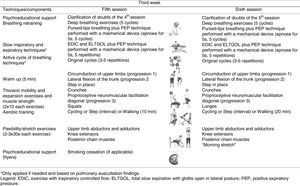
InterventionThe intervention consisted of standard medical treatment for the CG (i.e., pharmacotherapy) and standard medical treatment plus a community-based PR programme for the EG. The community-based PR programme was performed twice a week for 3 weeks.22,23 The mean duration of each session was 60min and included breathing control and airway clearance techniques, thoracic expansion and mobility exercises, exercise training and psychoeducational support.3,24,25 Exercise training was set at an intensity of 60–80% of patients’ maximum estimated heart rate and symptoms of dyspnoea and perceived exertion were maintained between 4 and 6 at the modified Borg scale (mBorg). Psychoeducational support was performed using flyers and verbal discussions.25,26 A multidisciplinary team was available to provide additional support. Sessions were held at the School of Health Sciences, University of Aveiro (ESSUA) or at patients’ home by a physiotherapist with experience in respiratory interventions. A detailed description of the intervention protocol can be found in Fig. 1.
Intervention protocol for the 3-week community-based pulmonary rehabilitation programme.
Sociodemographic (age, gender), anthropometric (height, weight, body mass index – BMI) and general clinical data (smoking habits, number of exacerbations in the past year, medication used in the stable and exacerbated period of the disease, long-term oxygen, non-invasive ventilation, comorbidities and physical activity levels) were collected within 48h of the AECOPD onset. The severity of comorbid diseases was recorded and scored according to the Charlson Comorbidity Index (CCI):27 mild (CCI scores of 1–2), moderate (CCI scores of 3–4) and severe (CCI scores ≥5). Physical activity level was assessed with the brief physical activity assessment tool.28,29 Additionally, severity of airflow limitation was classified based on the most recent spirometry from the patients’ clinical notes, performed when they were stable.1 Patients’ clinical notes were checked for any need of hospitalisation and/or unscheduled healthcare utilisation during the 3 weeks of intervention.
Outcomes assessed before and after the 3 weeks of intervention were dyspnoea during activities, quadriceps muscle strength, functionality and impact of the disease. Symptoms of dyspnoea and fatigue at rest, peripheral oxygen saturation (SpO2) and vital signs (i.e., respiratory and heart rates) were assessed before and after the intervention and also monitored in all PR sessions. All assessments were conducted by a trained physiotherapist following the standardised order described.
Dyspnoea and fatigue were assessed by asking participants to rate their perceived levels of these symptoms on the mBorg.30,31 The MCID established for dyspnoea symptoms in the mBorg is of 0.9 units for patients with AECOPD receiving pharmacological treatment.32 Symptoms of dyspnoea and fatigue, SpO2 and vital signs (to ensure patients’ security during the intervention7), were assessed at rest whilst participants were sitting and resting for at least 10min. Respiratory rate was assessed during 60s by direct observation of the chest wall.33 Heart rate and SpO2 were collected with a pulse oximeter (Pulsox 300i, Konica Minolta, Tokyo, Japan). In AECOPD, a SpO2<90% has been shown to have high sensitivity and specificity to detect both hypoxaemia (sensitivity=83.9%, specificity=88.9%) and hypercapnia (sensitivity=71.3%, specificity=76%),9,34 and has been positively correlated with arterial oxygen saturation (r=0.91; p<0.001).9
Dyspnoea during activities was assessed with the modified British Medical Research Council dyspnoea questionnaire (mMRC).35 This is a simple, valid and widely used instrument to characterise the impact of dyspnoea on the daily activities of patients with COPD that relates well with other measures of health status and predicts mortality risk.1 Variations of 0.6 units have been recently indicated as the minimal clinically important difference (MCID) for patients with AECOPD after pharmacological treatment.32
Quadriceps muscle strength was measured on the dominant side during an isometric contraction with a handheld dynamometer (microFET2, Hoggan Health, The best Salt Lake City, Utah).36 This is an important outcome since quadriceps muscle strength is an independent predictor of mortality in COPD37 and is usually decreased during AECOPD.38
Functionality was assessed with the five-repetition sit-to-stand test (5-STS) according to the protocol of Jones et al.39 A MCID of 1.7s has been established for patients with stable COPD after PR.39 According to the authors best knowledge, there is no MCID established for AECOPD.
Impact of the disease was measured with the COPD assessment test (CAT). CAT is one of the key outcome measures recommended by the GOLD1 to assess patients with COPD, and is also the outcome measure presenting more robust measurement properties during AECOPD.9 A MCID of 2 points for patients with AECOPD receiving pharmacological treatment has been established.40
Data analysisStatistical analyses were performed using IBM SPSS Statistics version 24.0 (IBM Corporation, Armonk, NY, USA) and plots created using GraphPad Prism version 5.01 (GraphPad Software, Inc., La Jolla, CA, USA). The level of significance was set at 0.05.
Descriptive statistics were used to describe the sample.
The normality of the data was explored with the Shapiro–Wilk test. Then, independent t tests, Mann–Whitney U tests and chi-squared tests were used to compare sociodemographic, anthropometric and general clinical characteristics between groups (i.e., EG vs. CG). The differences between pre- and post-intervention assessments, per group, were pooled for each outcome measure and Mann–Whitney U tests were used to compare groups. Comparisons between pre- and post-intervention assessments within each group were performed with Wilcoxon signed-rank tests.
Whenever possible, the number and percentage of participants in each group that improved above the MCID was determined and compared with chi-squared tests. Data are presented as mean±standard deviation or median [interquartile range].
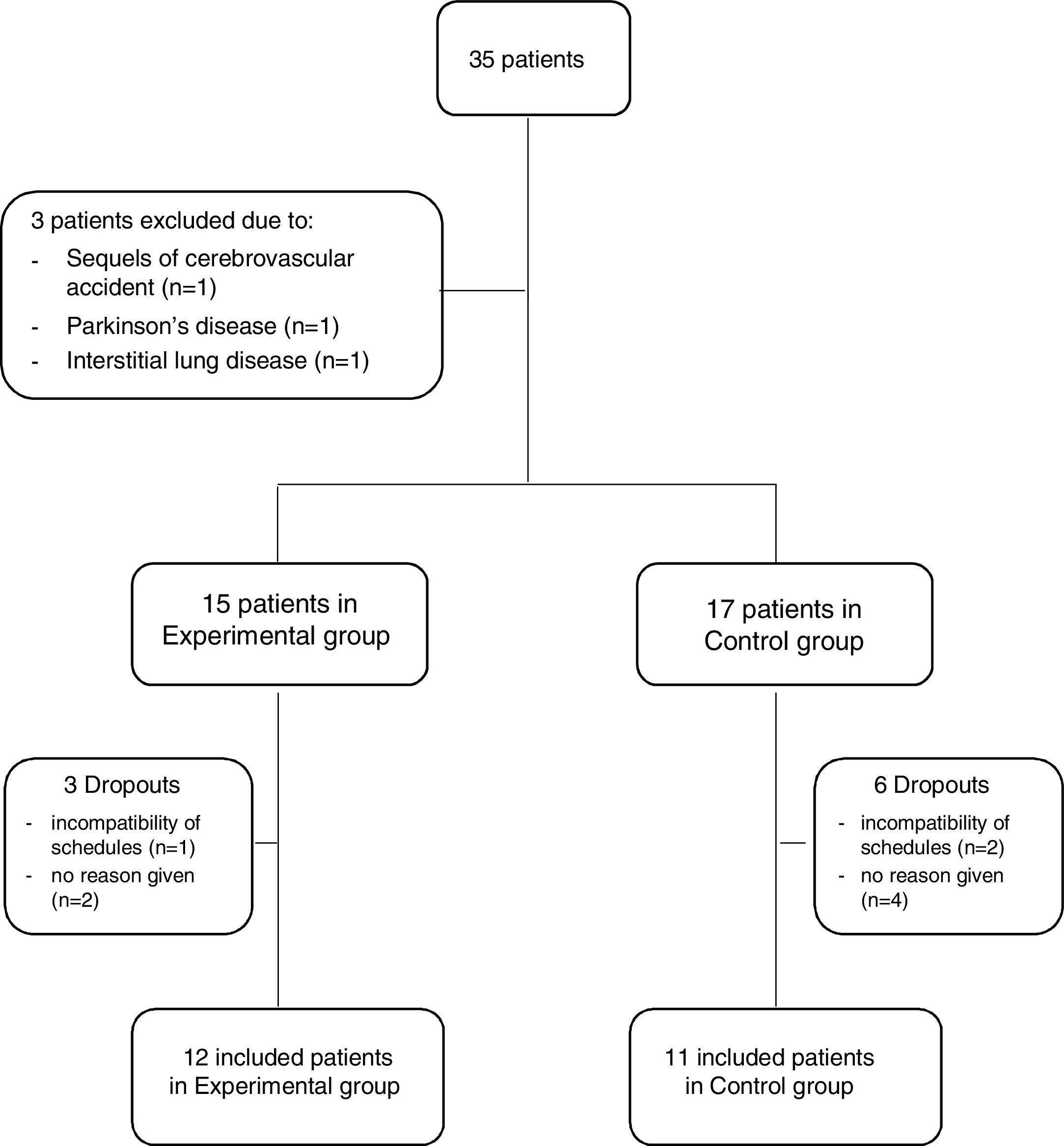
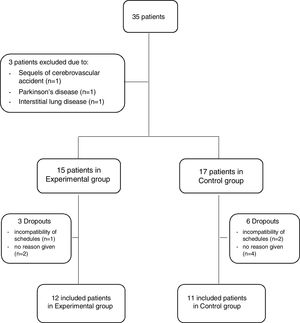
ResultsThirty-five outpatients with AECOPD were referred for possible inclusion in the study. From these, three were excluded due to the presence of sequels from a cerebrovascular accident that impaired her/his ability to perform the assessments (n=1), suffering from Parkinson's disease (n=1) and interstitial lung disease (n=1). Thus, 32 patients were invited to participate in the study and allocated to either the EG or CG. Three patients in the EG (1 due to incompatibility of schedules and 2 no reason given) and six in the CG (2 due to incompatibility of schedules and 4 no reason given) dropped out of the study. Therefore, 23 patients (19 males, 67.3±8.0 years, forced expiratory volume in 1s 57.2±23.9%predicted), 12 in the EG and 11 in the CG, were included (Fig. 2).
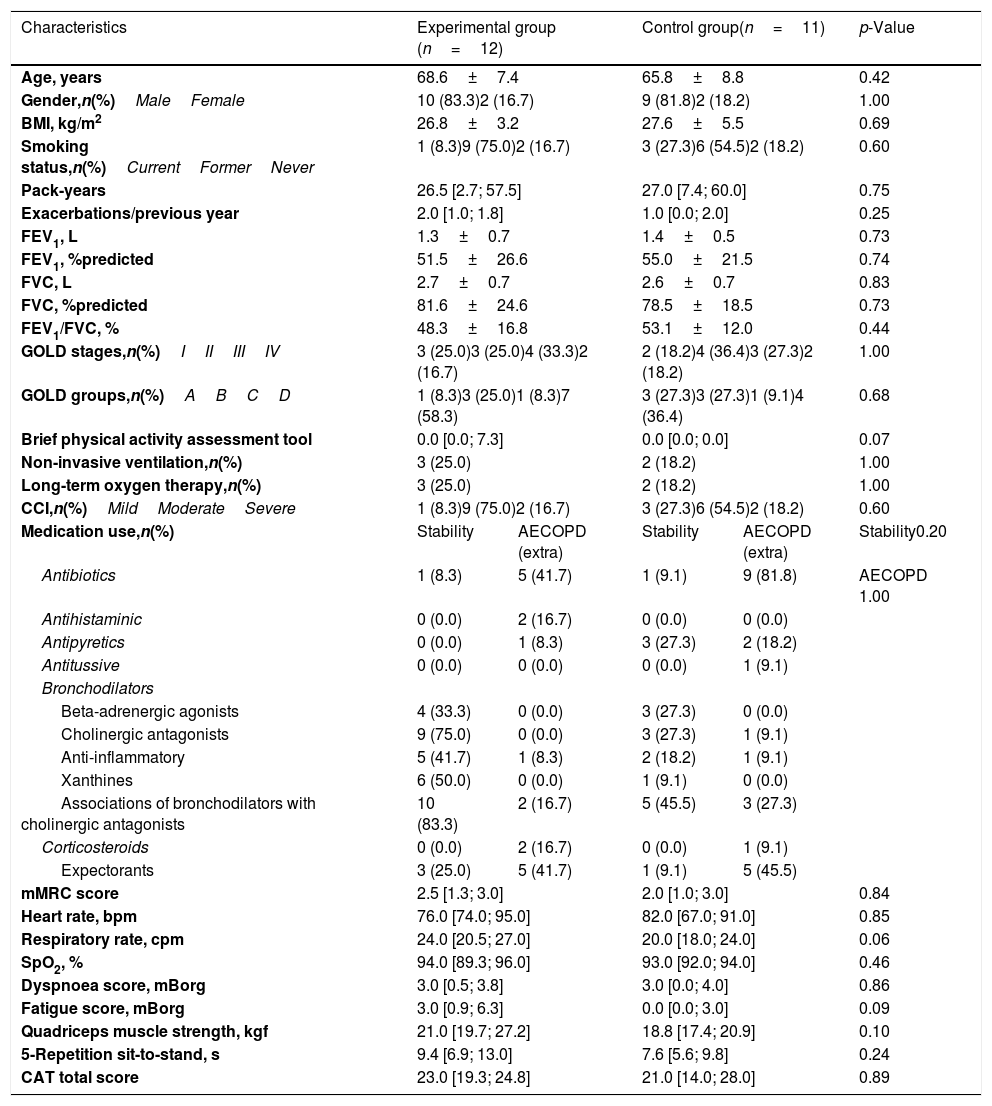
There were no significant differences between completers and dropouts in terms of age (p=0.551) and gender (p=0.303). Participants’ characteristics are summarised in Table 1. No significant differences were observed between groups at baseline (p>0.05).
Sample characterisation (n=23).
| Characteristics | Experimental group (n=12) | Control group(n=11) | p-Value | ||
|---|---|---|---|---|---|
| Age, years | 68.6±7.4 | 65.8±8.8 | 0.42 | ||
| Gender,n(%)MaleFemale | 10 (83.3)2 (16.7) | 9 (81.8)2 (18.2) | 1.00 | ||
| BMI, kg/m2 | 26.8±3.2 | 27.6±5.5 | 0.69 | ||
| Smoking status,n(%)CurrentFormerNever | 1 (8.3)9 (75.0)2 (16.7) | 3 (27.3)6 (54.5)2 (18.2) | 0.60 | ||
| Pack-years | 26.5 [2.7; 57.5] | 27.0 [7.4; 60.0] | 0.75 | ||
| Exacerbations/previous year | 2.0 [1.0; 1.8] | 1.0 [0.0; 2.0] | 0.25 | ||
| FEV1, L | 1.3±0.7 | 1.4±0.5 | 0.73 | ||
| FEV1, %predicted | 51.5±26.6 | 55.0±21.5 | 0.74 | ||
| FVC, L | 2.7±0.7 | 2.6±0.7 | 0.83 | ||
| FVC, %predicted | 81.6±24.6 | 78.5±18.5 | 0.73 | ||
| FEV1/FVC, % | 48.3±16.8 | 53.1±12.0 | 0.44 | ||
| GOLD stages,n(%)IIIIIIIV | 3 (25.0)3 (25.0)4 (33.3)2 (16.7) | 2 (18.2)4 (36.4)3 (27.3)2 (18.2) | 1.00 | ||
| GOLD groups,n(%)ABCD | 1 (8.3)3 (25.0)1 (8.3)7 (58.3) | 3 (27.3)3 (27.3)1 (9.1)4 (36.4) | 0.68 | ||
| Brief physical activity assessment tool | 0.0 [0.0; 7.3] | 0.0 [0.0; 0.0] | 0.07 | ||
| Non-invasive ventilation,n(%) | 3 (25.0) | 2 (18.2) | 1.00 | ||
| Long-term oxygen therapy,n(%) | 3 (25.0) | 2 (18.2) | 1.00 | ||
| CCI,n(%)MildModerateSevere | 1 (8.3)9 (75.0)2 (16.7) | 3 (27.3)6 (54.5)2 (18.2) | 0.60 | ||
| Medication use,n(%) | Stability | AECOPD (extra) | Stability | AECOPD (extra) | Stability0.20 |
| Antibiotics | 1 (8.3) | 5 (41.7) | 1 (9.1) | 9 (81.8) | AECOPD 1.00 |
| Antihistaminic | 0 (0.0) | 2 (16.7) | 0 (0.0) | 0 (0.0) | |
| Antipyretics | 0 (0.0) | 1 (8.3) | 3 (27.3) | 2 (18.2) | |
| Antitussive | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | |
| Bronchodilators | |||||
| Beta-adrenergic agonists | 4 (33.3) | 0 (0.0) | 3 (27.3) | 0 (0.0) | |
| Cholinergic antagonists | 9 (75.0) | 0 (0.0) | 3 (27.3) | 1 (9.1) | |
| Anti-inflammatory | 5 (41.7) | 1 (8.3) | 2 (18.2) | 1 (9.1) | |
| Xanthines | 6 (50.0) | 0 (0.0) | 1 (9.1) | 0 (0.0) | |
| Associations of bronchodilators with cholinergic antagonists | 10 (83.3) | 2 (16.7) | 5 (45.5) | 3 (27.3) | |
| Corticosteroids | 0 (0.0) | 2 (16.7) | 0 (0.0) | 1 (9.1) | |
| Expectorants | 3 (25.0) | 5 (41.7) | 1 (9.1) | 5 (45.5) | |
| mMRC score | 2.5 [1.3; 3.0] | 2.0 [1.0; 3.0] | 0.84 | ||
| Heart rate, bpm | 76.0 [74.0; 95.0] | 82.0 [67.0; 91.0] | 0.85 | ||
| Respiratory rate, cpm | 24.0 [20.5; 27.0] | 20.0 [18.0; 24.0] | 0.06 | ||
| SpO2, % | 94.0 [89.3; 96.0] | 93.0 [92.0; 94.0] | 0.46 | ||
| Dyspnoea score, mBorg | 3.0 [0.5; 3.8] | 3.0 [0.0; 4.0] | 0.86 | ||
| Fatigue score, mBorg | 3.0 [0.9; 6.3] | 0.0 [0.0; 3.0] | 0.09 | ||
| Quadriceps muscle strength, kgf | 21.0 [19.7; 27.2] | 18.8 [17.4; 20.9] | 0.10 | ||
| 5-Repetition sit-to-stand, s | 9.4 [6.9; 13.0] | 7.6 [5.6; 9.8] | 0.24 | ||
| CAT total score | 23.0 [19.3; 24.8] | 21.0 [14.0; 28.0] | 0.89 | ||
Values are presented as mean±standard deviation or median [interquartile range], unless otherwise stated.
Legend: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; BMI, body mass index; bpm, beats per minute; CAT, COPD assessment test; CCI, Charlson comorbidity index; cpm, cycles per minute; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; GOLD, Global Initiative for Chronic Obstructive Lung Disease; mBorg, modified Borg scale; mMRC, modified British Medical Research Council questionnaire; SpO2, peripheral oxygen saturation.
All patients in the EG completed the full PR programme (i.e., 6/6 sessions, attendance=100%) and no adverse events were reported.
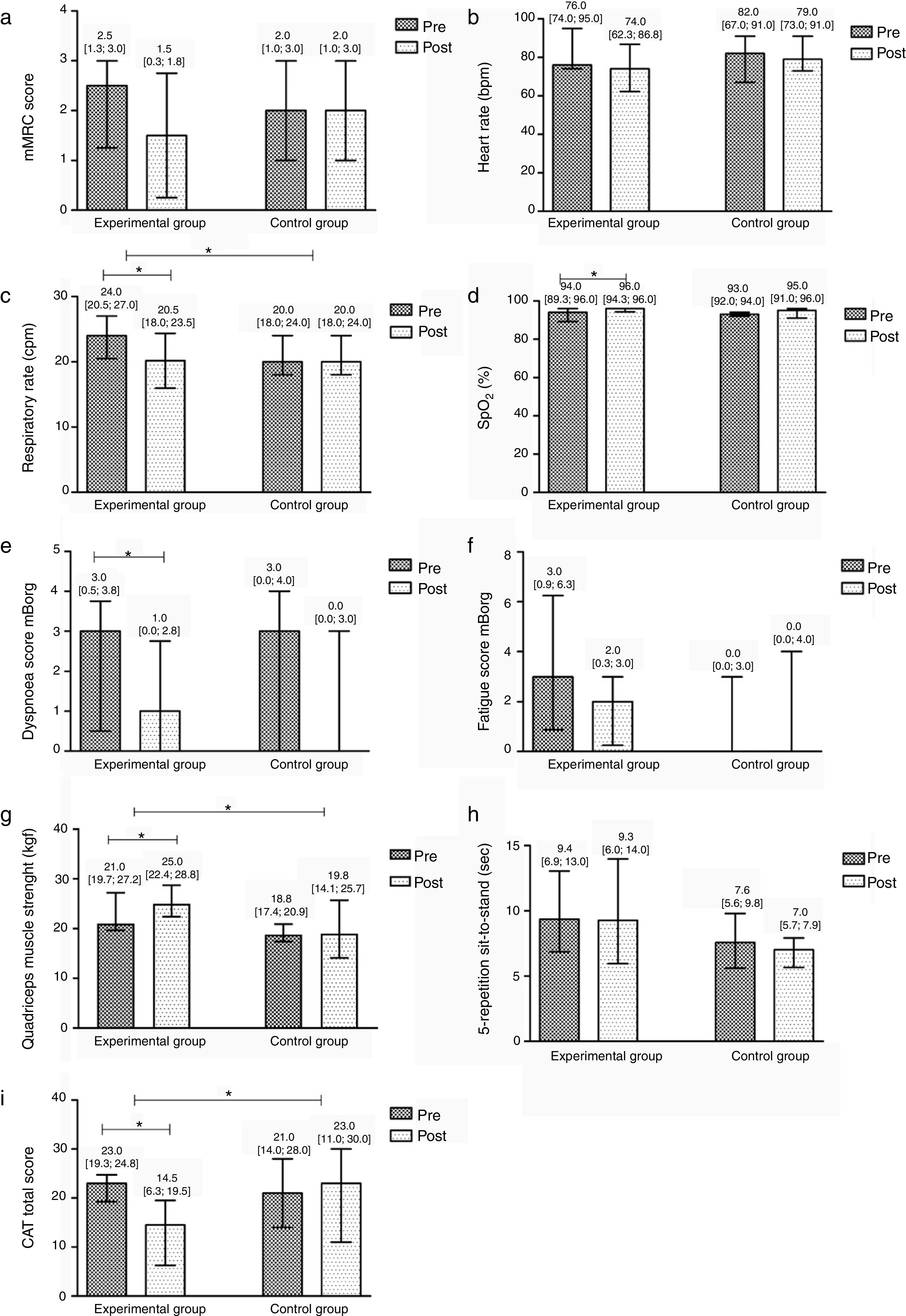
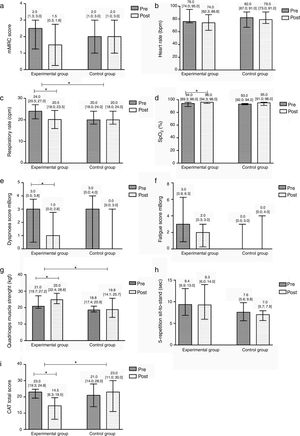
After the community-based PR programme, significant improvements were found in the EG in symptoms of dyspnoea at rest (Pre 3 [0.5; 3.8] vs. Post 1 [0.0; 2.8], p=0.008), respiratory rate (Pre 24 [20.5; 27] vs. Post 20.5 [18; 23.5], p=0.004), SpO2 (Pre 94 [89.3; 96] vs. Post 96 [94.3; 96], p=0.031), quadriceps muscle strength (Pre 21 [19.7; 27.2] vs. Post 25 [22.4; 28.8], p=0.012) and CAT score (Pre 23 [19.3; 24.8] vs. Post 14.5 [6.3; 19.5], p=0.008). No differences were found in the remaining outcome measures (p>0.05). The CG did not present any significant differences after the intervention (Fig. 3).
Median difference and interquartile range Pre/Post the intervention per group in: (a) the modified British Medical Research Council dyspnoea questionnaire (mMRC); (b) heart rate (beats per minute, bpm); (c) respiratory rate (cycles per minute, cpm); (d) peripheral oxygen saturation (SpO2); (e) symptoms of dyspnoea at rest in the modified Borg scale (mBorg); (f) symptoms of fatigue at rest in the modified Borg scale (mBorg); (g) quadriceps muscle strength; (h) the 5-repetition sit-to-stand test (5-STS); and (i) the COPD assessment test (CAT) total score.
In the between groups comparison, the EG showed significant improvements when compared to the CG in respiratory rate (EG −3.5 [−4; −0.5] vs. CG 2 [0; 4], p=0.015), quadriceps muscle strength (EG 3.1 [1.9; 7.2] vs. CG −0.5 [−2.8; 1.4], p=0.021) and CAT (EG −6 [−15; −4.0] vs. CG 0 [−8; 3], p=0.013). No additional differences were found (Fig. 3).
There was a significantly higher number of patients improving above the MCID of CAT in the EG than in the CG (EG 11 vs. CG 5, p=0.027). No differences were found for the remaining outcome measures.
None of the patients, either in the EG or in the CG, needed to be hospitalised or made unscheduled use of the healthcare services during the 3 weeks of intervention.
DiscussionThis quasi-experimental pilot study showed that community-based PR might be effective during mild-to-moderate AECOPD. Significant improvements were found in symptoms, vital signs, quadriceps muscle strength and impact of the disease and no adverse events were reported. However, patients were allowed to choose their group allocation.
Symptoms are key features of COPD41 and have been considered important outcomes of PR during exacerbations.3,42 In this study, after PR there was a significant decrease in dyspnoea levels at rest and most patients improved above the MCID in the mMRC and mBorg. Nevertheless, although positive improvements have been found for the EG, there were no significant differences in between group comparisons, which is probably related with the small sample size. Furthermore, no significant improvements have been found for fatigue, possibly because of the relatively low baseline fatigue scores found in our patients. These results must be carefully interpreted as dyspnoea and fatigue are multifactorial symptoms that should be described considered as both patients’ subjective perception and a performance component, and the mMRC and the mBorg are unidimensional tools that measure only a specific moment and were applied at rest.43,44 Therefore, these instruments might have failed to capture dyspnoea and the overall impact of fatigue. Multidimensional and disease-specific scales should be used in future studies to allow a multifactorial assessment of these symptoms and contribute to a better understanding of their behaviour during a PR programme in AECOPD.45 Moreover, the available MCIDs for the mMRC and the mBorg have been established for patients with AECOPD receiving standard of care.32 Future studies exploring MCIDs for patients with AECOPD undergoing PR are needed to better understand the added value of this intervention.
Despite the importance of assessing symptoms individually, it is their combination that impacts on patients’ HRQoL,9,41 hence a comprehensive symptom assessment is necessary.1 CAT is a short, simple, multidimensional and easy to administer questionnaire that has been developed to cover the most burdensome symptoms and limitations perceived by patients.46 Recent literature has shown that CAT is responsive to treatment47,48 and provides relevant prognostic information,49 therefore its use to assess PR during AECOPD has been advocated.9,47 In this study, the significant improvements and number of patients improving above the MCID show that CAT is sensitive to changes and supports its routine use in clinical practice.
Vital signs and SpO2 are measures previously used in PR programmes conducted in hospitalised patients with AECOPD and improvements, or patterns of improvement, have been reported,50–53 suggesting that PR is a safe approach during AECOPD. This pilot study corroborates these findings for PR implemented in the community. No adverse events were reported; however, a systematic assessment of adverse events was not conducted. Future studies should carefully assess occurrence of adverse events and the need for unscheduled healthcare visits during and after each session of PR to establish effectiveness and safeness of this intervention.
Quadriceps muscle weakness is a well-known systemic consequence of COPD1 that affects patients’ HRQoL and survival.54 During AECOPD there is a more accentuated decline in muscle function, resulting in long-term losses on functional capacity.55 This study, similarly to previous research, has shown that PR can prevent muscle dysfunction by improving quadriceps muscle strength during AECOPD,13,54,56,57 thus becoming a key intervention to manage peripheral muscle weakness and prevent further clinical decline.
It has been recognised that functionality, a vital outcome for patients’ daily life, is severely impaired during AECOPD.41,58 In this study, no differences were found in patients’ functionality after PR. This finding is likely to be due to the ceiling effect observed in the 5-STS and not to the ineffectiveness of the intervention. At baseline, patients already presented a good functional status as they were performing the test below the cut off for risk of falling (i.e., 12s),59 so with little room for improvement. Given the importance of this outcome, further research on the most appropriate outcome measure to assess functionality in patients with AECOPD is needed.
This study presents some limitations that need to be acknowledged. Firstly, patients were not randomised and assessors were not blinded. However, efforts were made to minimise the risk of bias by ensuring that patients characteristics at baseline were similar and implementing a well-defined assessment protocol and standardised intervention. Secondly, physical activity levels were not monitored during the AECOPD and it is possible that patients willing to participate in the PR programme were also more willing to be physically active. Nevertheless, baseline physical activity levels were no different between the groups. We registered however, a higher drop-out rate in the control group, probably due to patients’ lack of motivation60 and lack of perceived benefit of their participation.15 It is known that patients who have chosen not to attend PR are also less likely to participate in respiratory-related research61 and, thus, more likely to drop out. Third, pharmacological treatment was not standardised, but rather prescribed according to pulmonologists’ best judgement of patients’ clinical condition. Although no differences have been found regarding medication on baseline assessment, it must be acknowledged that different combinations of drugs might have influenced patients’ recovery. Fourth, exercise capacity, which is a key outcome of PR, was not assessed in this study due to lack of space to perform commonly used field exercise capacity tests (e.g., six-minute walk test and incremental shuttle walk test) at patients’ home, although aerobic training was performed. Future studies should explore the effects of community-based PR during AECOPD on patients’ exercise capacity using alternative field-tests. Since informative and promising results were obtained, there is now the need to adjust the outcome measures used and personalise PR to patients’ needs, in a randomised controlled trial.
ConclusionsCommunity-based PR seems feasible and offers promise to provide timely and effective management of patients with mild-to-moderate AECOPD. The addition of PR to standard of care resulted in improvements in respiratory rate, symptoms, quadriceps muscle strength and impact of the disease, parameters usually associated with an increased risk of AECOPD recurrence and poor prognosis,37,62,63 and no adverse events reported. Future research should focus on randomised studies with larger samples to clarify the role of community-based PR during AECOPD.
FundingThis work was funded by Programa Operacional de Competitividade e Internacionalização – POCI, through Fundo Europeu de Desenvolvimento Regional – FEDER (POCI-01-0145-FEDER-007628), Fundação para a Ciência e Tecnologia (PTDC/DTP-PIC/2284/2014 and PTDC/SAU-SER/28806/2017) and under the project UID/BIM/04501/2013 and UID/BIM/04501/2019.
Conflicts of interestThe authors report no conflict of interest.
The authors would like to acknowledge the assistance in data collection Cátia Paixão, Hélder Melro and Sara Miranda and the collaboration in this study of all patients and physicians.
Previous presentations: Part of this work was presented at the 28th European Respiratory Society International Congress as a poster discussion.