Asthma, a common chronic inflammatory disease, is treated with corticosteroid in most cases, but corticosteroid resistance in severe asthma patients seriously impairs the therapeutic effects. LncRNA-CASC7 inhibits cell proliferation and enhances drug sensitivity, but the molecular mechanisms of corticosteroid resistance in severe asthma are still unknown.
MethodsAirway smooth muscle cells (ASMCs) from healthy and severe asthmatic subjects were used in this study. The expression of CASC7 and miR-21 were modified by transfection with the pcDNA3.1-CASC7, miR-21 mimics and inhibitor. MTT assay was conducted to measure cell proliferation. ELISA assay was used to determine the secretion of CCL5, CCL11 and IL-6. The phosphorylation of glucocorticoid receptor (GR) and the PI3K/AKT signaling were assessed by western blotting assays. qRT-PCR was used to analyze the expression of CASC7, miR-21 and PTEN. Dual-luciferase reporter assay was used to assess the interaction among CASC7, miR-21 and PTEN.
ResultsCompared with AMSCs from severe asthma patients, dexamethasone inhibited cytokines (CCL5, CCL11 and IL-6) and promoted the phosphorylation of GR more significantly in normal AMSCs. CASC7 expression was suppressed while miR-21 expression and AKT activity were promoted in ASMCs from severe asthma patients. CASC7 promoted PTEN expression via directly inhibiting miR-21 expression. Overexpression of CASC7 suppressed the PI3K/AKT signaling pathway and promoted the inhibition effects of dexamethasone on cell proliferation and cytokines secretion via targeting miR-21.
ConclusionCASC7 increased corticosteroid sensitivity by inhibiting the PI3K/AKT signaling pathway via targeting miR-21, which provided a promising potential target for designing novel therapeutic strategy for severe asthma.
Asthma is a common chronic inflammatory disease with complex clinical symptoms, including coughing, chest tightness and breathlessness, affecting approximately 300 million people of all ages worldwide.1 Asthma is characterized by airway inflammation, bronchospasm and airflow obstruction. Eosinophils, T cells, airway smooth muscle cells (ASMCs) and epithelial cells are contributing to the pathogenesis of asthma.2 Although there is still no cure for asthma, fortunately for most patients, it can be managed and controlled to prevent exacerbations and reduce the risk of mortality.3 Glucocorticoid is widely used to treat asthma, and has excellent effects for easing asthma symptoms.4 However, approximately 5–10% of asthma suffers respond poorly to corticosteroid treatment even with high doses of corticosteroid, which is termed “corticosteroid resistance”.5 Corticosteroid resistance is defined by the failure to improve the airway obstruction even after an oral corticosteroid therapy for 2 weeks.6 Advances in investigating the molecular mechanisms of corticosteroid resistance can help us have a better understanding of the pathogenesis of asthma with different corticosteroid sensitivity and how we can develop new agents to treat asthma patients with severe corticosteroid resistance.
With the achievements in studying the molecular mechanisms of corticosteroid resistance, some mechanisms have been identified, including the involvement of microRNAs (miRNAs). Several miRNAs were dysregulated in asthma patients and mouse models, such as miR-1, miR-21, miR-126 and miR-221.7 Of these, miR-21 was up-regulated, promoted corticosteroid resistance and might be used as a new biomarker for diagnosis in asthma.8,9 However, the up-stream regulatory mechanism and down-stream targets of miR-21 in corticosteroid resistant asthma still remain largely unknown. miRNAs are generally considered as mediators of long non-coding RNAs (lncRNAs) to regulate gene expression. As reported, lncRNA-CASC7 was demonstrated to suppress the migration and proliferation of colon cancer cells by inhibiting miR-21,10 but the regulation of miR-21 by CASC7 in asthma still needs to be clarified.
The PI3K/AKT signaling pathway was essential for the airway smooth muscle contraction and associated with corticosteroid insensitivity by suppressing HDAC-2 activity.11 In addition, PTEN was down-regulated in asthma and identified to block the activity of PI3K by dephosphorylating.12,13 It was reported that miR-21 inhibited PTEN expression and promoted PI3K activity in lung tissues of steroid-insensitive asthmatic mice,8 but the role of miR-21 in severe asthma patients and its regulatory way of PTEN/PI3K/AKT pathway are still not very clear. Furthermore, we found a binding site for miR-21 on CASC7 and 3′-untranslated region (UTR) of PTEN by bioinformatics analysis. Therefore, we presumed that, in patients with corticosteroid resistant asthma, CASC7 might directly target miR-21 to up-regulate PTEN expression and then increase the sensitivity of ASMCs to glucocorticoid by inhibiting the PI3K/AKT signaling pathway.
In this study, we found that CASC7 expression was suppressed and miR-21 was up-regulated in ASMCs from severe asthma patients. CASC7 could promote PTEN expression by directly binding to miR-21, thereby inhibiting PI3K/AKT signaling pathway. Overexpression of CASC7 could promote the inhibitory effect of dexamethasone on cell proliferation and cytokine release, and promote glucocorticoid receptor (GR) phosphorylation in ASMCs from severe asthma patients. Our results provide new evidence to help elucidate the molecular mechanisms of corticosteroid resistance in asthma and suggest CASC7 may be a promising potential target for designing novel clinical therapeutic strategy for severe asthma patients.
MethodsCell isolation, culture and treatmentASMCs were isolated and cultured from bronchial biopsies from healthy (n=6) and severe asthmatic (n=6) subjects as previously described.14 Briefly, bronchial biopsies were cut into small pieces and allowed to attach on culture plates in Dulbecco's modified Eagle's medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific), 20μg/mL streptomycin (Thermo Fisher Scientific), 20U/L of penicillin (Thermo Fisher Scientific), 4mM L-glutamine (Thermo Fisher Scientific) and 2.5μg/mL amphotericin B (Thermo Fisher Scientific), to allow ASMC growth at 37˚C in a humidified incubator supplemented with 5% CO2. Written informed permission was obtained from the asthma patients and healthy donors. This study was approved by the Ethics Committee of the First Affiliated Hospital of Hebei North University. The human 293T cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), and was maintained in DMEM supplemented with 10% FBS, 100U/ml penicillin, 100μg/ml streptomycin at 37˚C in a humidified incubator supplemented with 5% CO2.
Different concentration of dexamethasone (10−10, 10−9, 10−8, 10−7 and 10−6M, Sigma-Aldrich, St. Louis, MO, USA) were directly added into ASMC culture for 2h. For inducing cell proliferation and inflammatory factor secretion, TNF-α was added at 10ng/mL and then incubated for 24h.
Cell transfectionCASC7 sequence was synthesized and sub-cloned into transfection plasmid to generate recombinant vector pcDNA3.1-CASC7 (CASC7), and the sequence was verified. miR-21 mimics, miR-21 inhibitor and the corresponding control mimics (mimics NC) or control inhibitor (inhibitor NC) were purchased from GenePharma (Shanghai, China). Cell transfection and co-transfection were conducted with Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific) following the manufacture's instruction. After 48h, cells were harvested for subsequent experiments.
Dual-luciferase reporter assayTo construct dual luciferase reporter plasmids, the predicted binding sequence of miR-21 in CASC7 (WT-CASC7) and PTEN (WT-PTEN) and their mutated sequence (MUT-CASC7/PTEN) were separately cloned into pmirGLO vector (Promega, Madison, WI, USA). For luciferase assay, 293T cells were transfected with above constructs and co-transfected with miR-21 mimics, inhibitor or the corresponding control. After 48h, 293T cells were harvested, and the luciferase activities were measured using the Dual-Glo® Luciferase Assay System (Promega) according to the manufacturer's direction. The relative firefly luciferase activity was calculated by normalizing to renilla luciferase activity.
MTT assayMTT assay was conducted to measure the proliferation of AMSCs. Briefly, AMSCs were seeded into 96-well plate with 1×104cells per well. After 24h, the medium was replaced with fresh culture medium. Then 10μL of MTT stock solution (Thermo Fisher Scientific) was added to the cells, and incubated at 37°C for 4h. Then, the supernatants were discarded carefully and 100μL dimethyl sulfoxide were added to dissolve the formazan crystals. The absorbance of each well at 450nm was recorded using a microplate reader (Bio-Tek Instruments, Winooski, VT, USA).
Enzyme-linked immunosorbent assay (ELISA)The concentrations of CCL5, CCL11 and IL-6 in culture supernatant of ASMCs after TNF-α treatment and/or indicated transfection were determined using ELISA. In brief, AMSCs were treated or transfected differently, and the culture supernatant was collected. The concentrations of CCL5, CCL11 and IL-6 in the culture supernatant were determined using ELISA kits obtained from Thermo Fisher Scientific following the product manual.
RNA isolation and quantitative real-time PCR (qRT-PCR)Total RNA was extracted from ASMCs using TRIzol reagent (Thermo Fisher Scientific) and was reverse-transcribed into cDNA using a PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's directions. For miR-21, miRNA was extracted from cells with mirVana™ miRNA Isolation Kit (Thermo Fisher Scientific), and was reverse transcribed into cDNA using TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA). For CASC7, high-capacity RNA to cDNA kit (Applied Biosystems) was used to synthesize cDNA after total RNA extraction. Expression levels of indicated genes, miR-21, CASC7 and PTEN, were detected using Applied Biosystems 7500 Real Time PCR System (Applied Biosystems) with a SYBR Green QPCR Master Mix (Thermo Fisher Scientific). Primers used in PCR were listed below: CASC7 forward 5′-ATCAACGTCAAGCTGGGAGG-3′, reverse 5′-CTTGTCCCCCGCTCGTTC-3′; miR-21 forward 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACG ACTCAACA-3′, reverse 5′-CGCGCTAGCTTATCAGACTGA-3′; PTEN forward 5′-TTACAGTTGGGCCCTGTACC-3′, reverse 5′-ATTTGATGCTGCCGGTAAAC-3′; U6 (internal control for miRNA) forward 5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-AACGCTTCACGAATTTGCGT-3′; GAPDH (internal control for mRNAs) forward 5′-CCAGGTGGTCTCCTCTGA-3′ and reverse 5′-GCTGTAGCCAAATCGTTGT-3′.The relative RNA levels were calculated by the 2−ΔΔCt method. U6 and GAPDH were used as internal controls.
Western blottingASMCs lysates were prepared in RIPA buffer (Thermo Fisher Scientific, MA, USA), and protein concentrations were determined using BCA protein quantitation kit (Thermo Fisher Scientific). 30μg cell lysates were subjected to 10% SDS-PAGE electrophoresis per lane and transferred to PVDF membrane (GE Healthcare, Pittsburgh, PA, USA). The blots were blocked with 5% non-fat milk for 1h, and probed with primary antibody against phosphorylated GR (1:1000), GR (1:1000), GAPDH (1:5000), PTEN (1:2000), p-AKT (1:1000) and AKT (1:1000) overnight at 4°C. All primary antibodies were ordered from Cell Signaling Technology (Boston, MA, USA). Then, the membranes were washed three times with TBST buffer prior to incubation with HRP linked secondary antibodies (Thermo Fisher Scientific), and were visualized using an ECL detection system (Thermo Fisher Scientific). The relative densities of bands were analyzed with NIH image J software.
Statistical analysisAll experiments were conducted at least three times in triplicate, and one representative experiment was shown. All data shown were expressed as mean±standard deviation (SD). Statistical analysis was performed using the two-sided Student's t-test for comparison between two independent groups or one-way analysis of variance (ANOVA) followed by Tukey post hoc test for multiple comparison with Graphpad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). p-Values <0.05 were considered statistically significant.
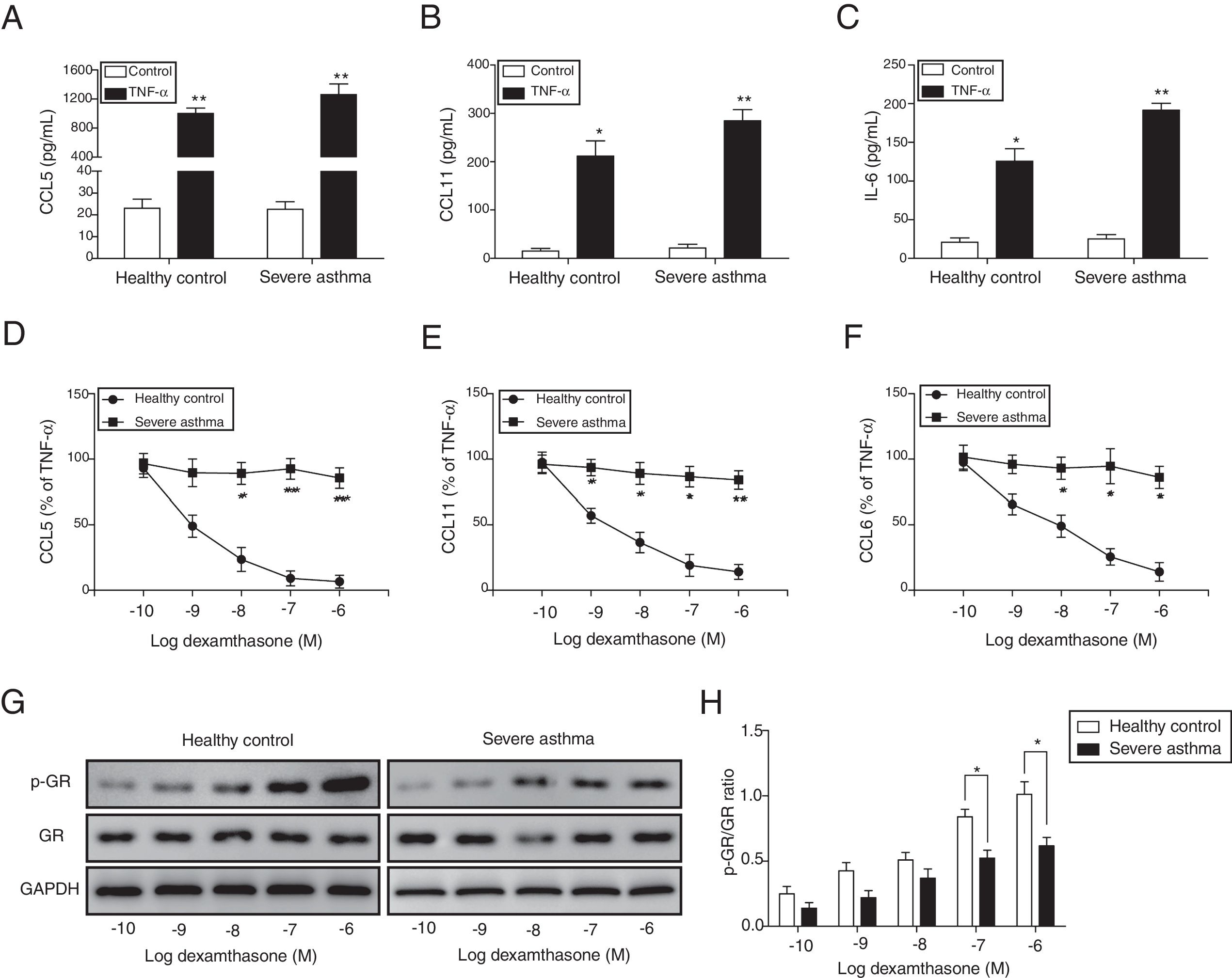
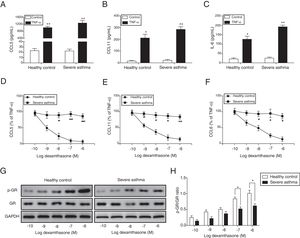
ResultsThe corticosteroid sensitivity was impaired in ASMCs from severe asthma patientsTo investigate the corticosteroid sensitivity of ASMCs, we isolated and cultured ASMCs from healthy and severe asthmatic subjects separately. After TNF-α treatment, ELISA assays were used to analyze the production of chemokines and inflammatory cytokines, and all these factors (CCL5, CCL11 and IL-6) were up-regulated, especially in severe asthma patient cells with more induction of these factors (Fig. 1A–C). Then we treated ASMCs at different concentration of dexamethasone after TNF-α treatment, and we found that dexamethasone effectively inhibited the secretion of CCL5, CCL11 and IL-6 in normal ASMCs in concentration dependent manner, but not in the ASMCs from severe asthma patients, indicating the corticosteroid sensitivity was impaired in ASMCs from severe asthma patients (Fig. 1D–F). As GRs become hyper-phosphorylated upon glucocorticoid treatment, we examined the phosphorylation level of GR in ASMCs after treatment. As shown in Fig. 1G&H, dexamethasone markedly promoted the phosphorylation of GR in normal ASMCs compared with ASMCs from severe asthma patients was impaired. These results suggested ASMCs from severe asthma patients respond poorly to dexamethasone treatment.
ASMCs from severe asthma patients respond poorly to dexamethasone treatment. The secretion of CCL5 (A), CCL11 (B) and IL-6 (C) was detected by ELISA assay in ASMCs culture supernatant from healthy or severe asthmatic subjects after TNF-α treatment. The secretion of CCL5 (D), CCL11 (E) and IL-6 (F) was measured by ELISA assay in ASMCs culture supernatant after dexamethasone and TNF-α treatment. (G) The protein level of p-GR and GR was detected by western blot analysis in ASMCs treated with different concentration dexamethasone. GAPDH was used a normalization control. (H) The p-GR/GR ratio was quantified. All the results were shown as mean±SD (n=3), and two-sided Student's t-test was used for statistical analysis. *p<0.05 and **p<0.01.
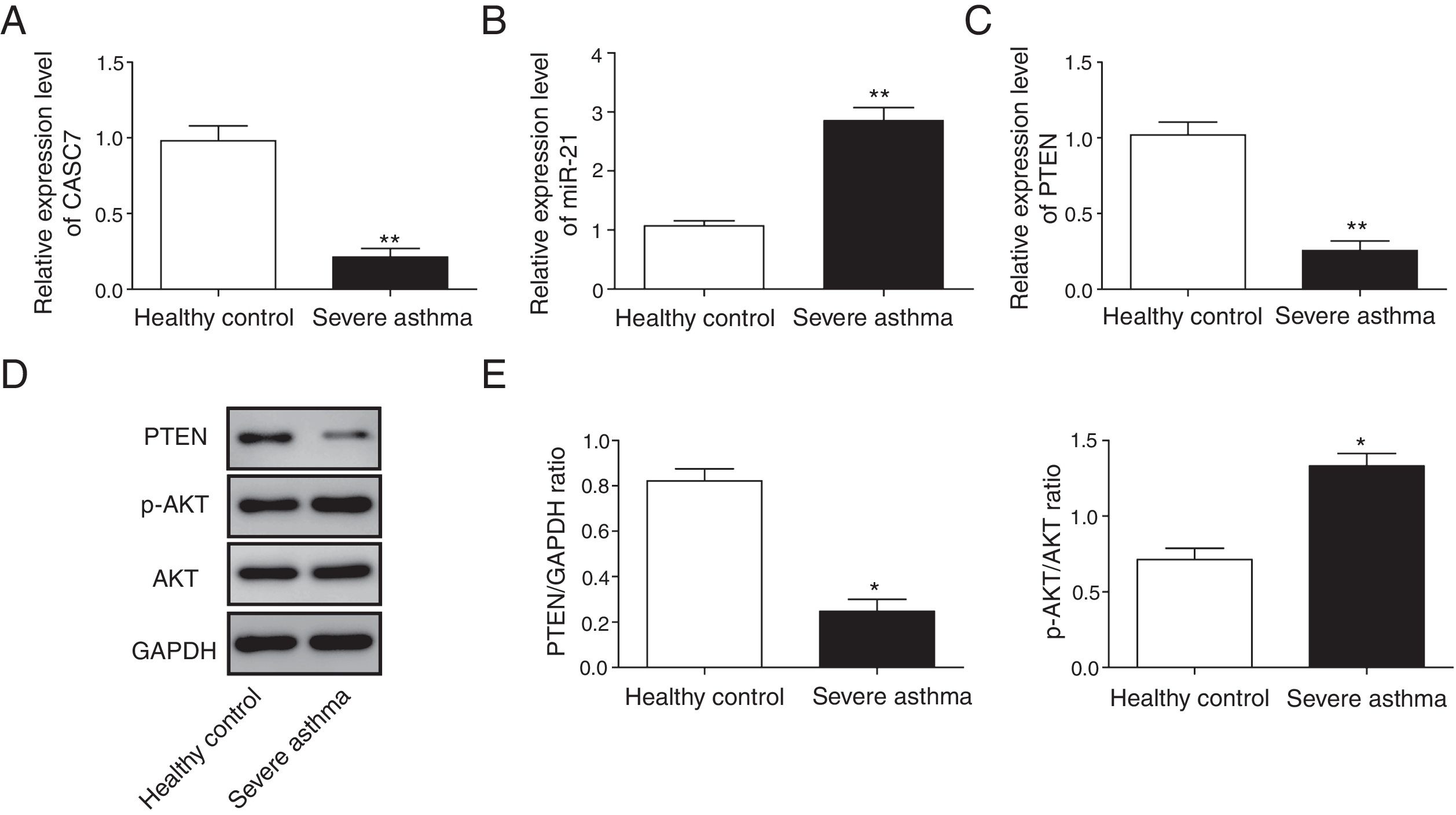
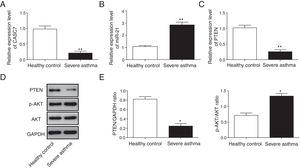
Next, we examined the expression level of CASC7, miR-21 and PTEN in ASMCs from severe asthma patients. We found that CASC7 was down-regulated in ASMCs from severe asthma patients compared with normal ASMCs (Fig. 2A), providing the possible link between CASC7 and corticosteroid insensitivity. Furthermore, the expression of miR-21 was significantly up-regulated in severe asthma ASMCs, which was negatively correlated with CASC7 expression (Fig. 2B). Because miR-21 was reported to modulate PI3K/AKT activity via regulating PTEN expression, we analyzed PTEN expression and AKT activity. PTEN expression was decreased in severe asthma ASMCs at both mRNA and protein levels (Fig. 2C–E), and AKT phosphorylation was increased in severe asthma ASMCs (Fig. 2D&E). These observations implied that CASC7 might be involved in maintaining corticosteroid sensitivity possibly via regulating miR-21/PTEN axis and the PI3K/AKT signaling pathway.
CASC7 and PTEN were down-regulated while miR-21 was up-regulated in ASMCs from severe asthma patients. The expression level of CASC7 (A), miR-21 (B) and PTEN (C) was detected by qRT-PCR in ASMCs from healthy or severe asthmatic subjects. (D) The protein levels of PTEN, p-AKT and AKT were assessed by western blot analysis in ASMCs from healthy or severe asthmatic subjects. GAPDH was used a normalization control. (E) The ratio of PTEN/GAPDH and p-AKT/AKT was quantified. All the results were shown as mean±SD (n=3), and two-sided Student's t-test was used for statistical analysis. *p<0.05 and **p<0.01.
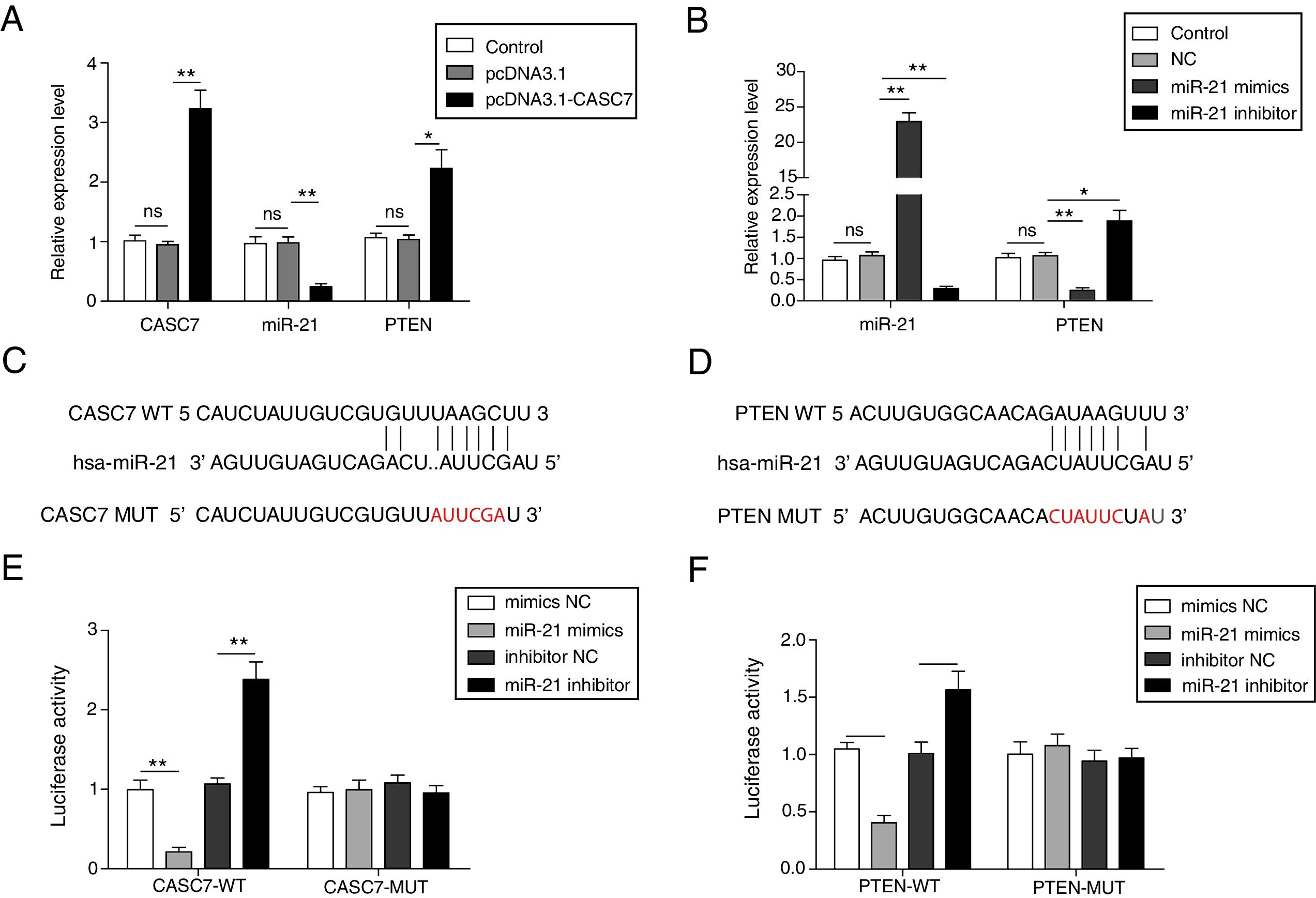
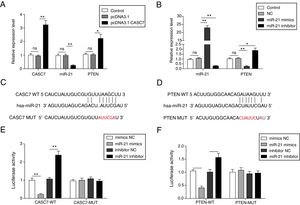
To investigate the regulatory relationship among CASC7, miR-21 and PTEN, AMSCs were transfected with pcDNA3.1-CASC7. We found CASC7 and PTEN were highly up-regulated, and miR-21 expression was obviously decreased in ASMCs transfected with pcDNA3.1-CASC7 (Fig. 3A). As shown in Fig. 3B, miR-21 mimics resulted in highly expression of miR-21 and markedly reduced expression of PTEN. Conversely, miR-21 expression was suppressed and PTEN expression was up-regulated in ASMCs transfected with miR-21 inhibitor (Fig. 3B). These data indicated that CASC7 was very likely to regulated PTEN expression via modulating miR-21 expression. To further validate the interaction among CASC7, miR-21 and PTEN, we predicted that there were binding sites of miR-21 in CASC7 and the 3′-UTR of PTEN by bioinformatic analysis (Fig. 3C&D). Dual-luciferase reporter assays showed that the luciferase activity was markedly decreased by co-transfection with miR-21 mimics and wild type CASC7 construct (CASC7-WT), while the enhanced luciferase activity was observed by co-transfection with miR-21 inhibitor and WT-CASC7, suggesting that miR-21 was the direct target of CASC7 (Fig. 3E). The luciferase activity was not changed with mutated CASC7 construct (CASC7-MUT) (Fig. 3E). Similar results were also observed in PTEN-WT and PTEN-MUT (Fig. 3F). These observations demonstrated that CASC7 could directly sponge miR-21 to counteract its suppression on PTEN, serving as a positive regulator of PTEN.
CASC7 increased PTEN expression via targeting miR-21. (A) The expression level of CASC7, miR-21 and PTEN was detected by qRT-PCR in ASMCs transfected with pcDNA3.1-CASC7. (B) The expression level of miR-21 and PTEN was assessed by qRT-PCR in ASMCs transfected with miR-21 mimics or miR-21 inhibitor. (C) The predicted miR-21 binding site within CASC7. (D) The predicted binding of miR-21 and 3′-UTR of PTEN. The luciferase reporter containing the predicted binding sties of miR-21 within CASC7 (E) and PTEN (F) were co-transfected with miR-21 mimic or inhibitor, and then the luciferase activity was measured by dual luciferase assay. All the results were shown as mean±SD (n=3), and ANOVA was conducted for statistical analysis. *p<0.05 and **p<0.01.
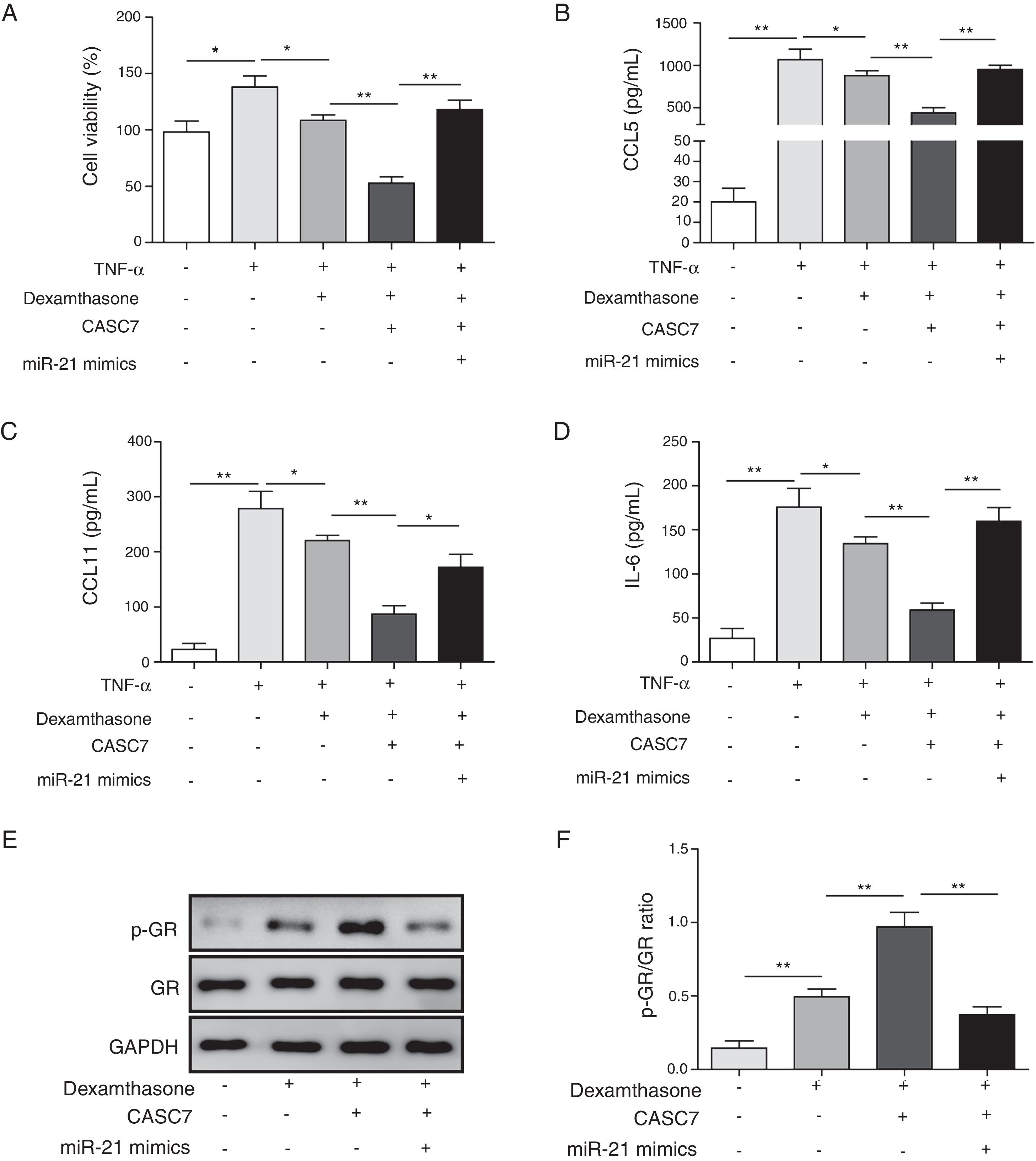
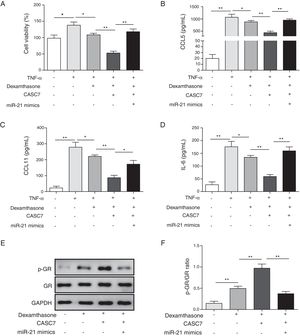
After ASMCs were transfected with pcDAN3.1-CASC7 and miR-21 mimics, the effects of dexamethasone on cell proliferation and cytokine secretion were examined by MTT and ELISA assay, respectively. Compared with control group, overexpression of CASC7 promoted dexamethasone-induced ASMCs proliferation inhibition, but was reversed by miR-21 mimics transfection (Fig. 4A). The secretion of CCL5, CCL11 and IL-6 was suppressed by overexpressing CASC7, which were also partially reversed by miR-21 mimics transfection (Fig. 4B–D). In addition, overexpression of CASC7 promoted the phosphorylation of GR induced by dexamethasone, and it was restored by miR-21 mimics (Fig. 4E&F). These results indicated that CASC7 could suppress the proliferation and the secretion of CCL5, CCL11 and IL-6 by repressing miR-21 expression, thereby increasing the sensitivity of ASMCs to corticosteroids.
Overexpression of CASC7 promoted dexamethasone induced the inhibition of cytokine release and GR phosphorylation via decreasing miR-21. (A) Cell proliferation was detected by MTT assay in ASMCs. The secretion of CCL5 (B), CCL11 (C) and IL-6 (D) was assessed by ELISA assay in ASMC culture supernatant. (E) The protein level of p-GR and GR was measured by western blot analysis in ASMCs. GAPDH was used a normalization control. (F) The ratio of p-GR/GR was quantified. All the results were shown as mean±SD (n=3), and ANOVA was conducted for statistical analysis. *p<0.05 and **p<0.01.
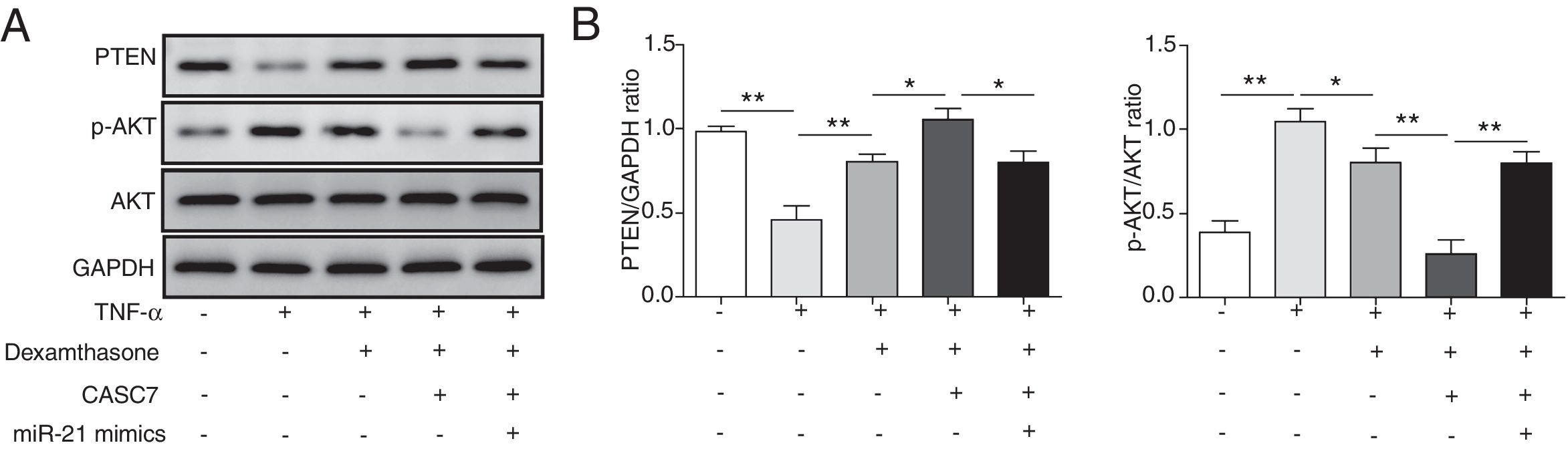
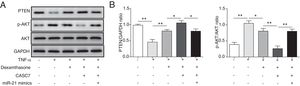
Since PTEN was identified to block the activity of the PI3K/AKT signaling by dephosphorylating,12 we examined whether overexpression of CASC7 affected the PI3K/AKT signaling pathway by western blotting. In ASMCs, TNF-α treatment could decrease PTEN expression while dexamethasone could inhibit TNF-α induced PTEN down-regulation (Fig. 5A&B). Overexpression of CASC7 facilitated the above inhibition of dexamethasone by directly down-regulating miR-21 (Fig. 5A&B). In contrast with the expression pattern of PTEN, dexamethasone could suppress the phosphorylation of AKT induced by TNF-α, which was promoted by overexpressing CASC7 (Fig. 5A&B). Moreover, miR-21 mimics restored the high level of AKT phosphorylation (Fig. 5A&B). These results provided the evidence that CASC7 might attenuate corticosteroid resistance by targeting miR-21 to inhibit the PI3K/AKT signaling pathway.
CASC7 facilitated inhibition of the PI3K/AKT signaling pathway by dexamethasone via inhibiting miR-21. (A) The protein level of PTEN, p-AKT and AKT was measured by western blot analysis in ASMCs. GAPDH was used a normalization control. (B) The ratio of PTEN/GAPDH and p-AKT/AKT was quantified. All the results were shown as mean±SD (n=3), and ANOVA was conducted for statistical analysis. *p<0.05 and **p<0.01.
Corticosteroids are the main therapy for asthma worldwide, and effectively ease the symptoms of asthma.15 Although it is an effective treatment for patients with mild to moderate asthma, there are still 5–10% severe asthma patients respond poorly to corticosteroids, which leads to a high socioeconomic burden and impairs the quality of life of patients at high dose of corticosteroids.16 Therefore, it is very important to elucidate the molecular mechanisms of corticosteroid resistance. In this study, we firstly demonstrated that CASC7 promoted the corticosteroid sensitivity by targeting miR-21 to repress the PI3K/AKT signaling pathway, which provide new understanding of mechanisms of corticosteroid resistance.
LncRNAs regulate gene expression by various mechanisms and have emerged as a key modulator in regulating physiological processes.17 LncRNAs play important roles in asthma, and might be associated with corticosteroid resistance.18 For example, lncRNA-GAS5 was reported to serve as a potential regulator of corticosteroid resistance via acting as a decoy for GR.19 LncRNA-PVT1 was up-regulated in patients with corticosteroid resistant severe asthma, indicating it might be involved in the regulation of corticosteroid resistance.20 CASC7 is an approximately 9.3kb dual-localized lncRNA whose function is largely unknown,21 especially in corticosteroid resistant asthma. Here we found CASC7 was down-regulated while miR-21 was up-regulated in severe asthma ASMCs compared with normal cells, indicating the possible involvement of CASC7 and miR-21 in maintaining corticosteroid sensitivity. CASC7 was down-regulated colon cancer, and overexpression of CASC7 inhibited cell proliferation and migration by regulating miR-21.10 We observed the up-regulation of miR-21 in severe asthma ASMCs, which also has been reported in asthmatic children.22 Based on these results, we demonstrated for the first time that lncRNA-CASC7 directly binds to miR-21 to regulate corticosteroid resistance in severe asthma.
PTEN is a phosphatase acting as a negative regulator of the PI3K/AKT signaling.23 PI3K is activated by tyrosine kinases, and then phosphorylates PIP2 to generate PIP3, and then PIP3 recruits AKT to membrane where it is phosphorylated and activated.24,25 PTEN suppresses the PI3K/AKT signaling by converting PIP3 back to PIP2.26 The PTEN/PI3K/AKT signaling pathway played multifaceted roles in asthma pathogenesis.27 Consistent with the reports that miR-21 activated the PI3K/AKT pathway28 and CASC7 directly targeted miR-21,10 we found CASC7 promoted the expression of PTEN and inhibited the PI3K/AKT signaling via directly suppressing miR-21 expression.
In human asthma, CCL5 and CCL11 are secreted at high levels, and associated with corticosteroid insensitivity.29 As reported, dexamethasone or fluticasone treatment did not affect the production of CCL5, CCL11 and IL-6 in ASMCs from severe asthma patients compared to healthy controls.30,31 GR is ligand-dependent transcription factors to regulate gene transcription, and the phosphorylation of GR is the main pharmacological mechanism of corticosteroid for treating inflammatory diseases including asthma.32 GR are phosphorylated upon hormone binding, which is important for GR to exert its regulatory function.33 We also found the phosphorylation level of GR was significantly reduced and CCL5, CCL11 and IL-6 were less easily suppressed by dexamethasone treatment in severe asthma ASMCs compared with healthy controls. However, the molecular mechanisms of regulating GR phosphorylation need more investigation.
Although CASC7 was r previously reported to target miR-21 in colon cancer cells,10 it was the first evidence to reveal the involvement of CASC7 in severe asthma and the regulation of miR-21 by CASC7 in ASMCs. Additionally, previous studies showed that in lung tissue of asthmatic mice, the expression of PTEN was negatively regulated by miR-21,8 but the upstream regulatory mechanism of miR-21 in severe asthma patients with corticosteroid resistance and its regulatory way of PTEN are unclear. This study demonstrated that CASC7 promoted PTEN expression to inhibit PI3K/AKT signaling pathway by sponging miR-21 in ASMCs from severe asthma patients. Taken together, our results suggested that CASC7 might maintain corticosteroid sensitivity by directly regulating miR-21/PTEN axis to inhibit the PI3K/AKT signaling pathway in ASMCs for the first time. Overexpression of CASC7 promoted sensitivity of ASMCs to corticosteroids by directly down-regulating miR-21. Our work provides deeper insights for CASC7 in severe asthma and may provide new potential targets for corticosteroid resistance.
Conflict of interestThe authors have no conflicts of interest to declare.
This work was supported by Hebei Provincial Science and Technology Department's Self-financing Project of the 2016 Science and Technology Support Plan (No. 162777297).