Rheumatoid arthritis (RA) is associated with several extra-articular manifestations, including lung disease. Pulmonary manifestations can result from parenchyma, airways, pleura or vasculature involvement and are a major contributor to morbimortality.1
Follicular bronchiolitis (FB) is a small airways disease reported in RA patients, which results from hyperplasia of bronchus-associated lymphoid tissue.
We report the case of a 49-years-old Caucasian female, who presented with an erosive polyarthritis of hands and wrists at the age of 25, with positive anti-citrullinated protein antibodies and negative rheumatoid factor. A diagnosis of RA was assumed, without extra-articular involvement. She had previously received hydroxychloroquine (stopped for retinopathy), infliximab and adalimumab (both stopped due to adverse reaction) and etanercept (stopped for inefficacy) and was currently under prednisolone (PDN) 5mg/day and methotrexate (MTX) 20mg/week, after one cycle of rituximab.
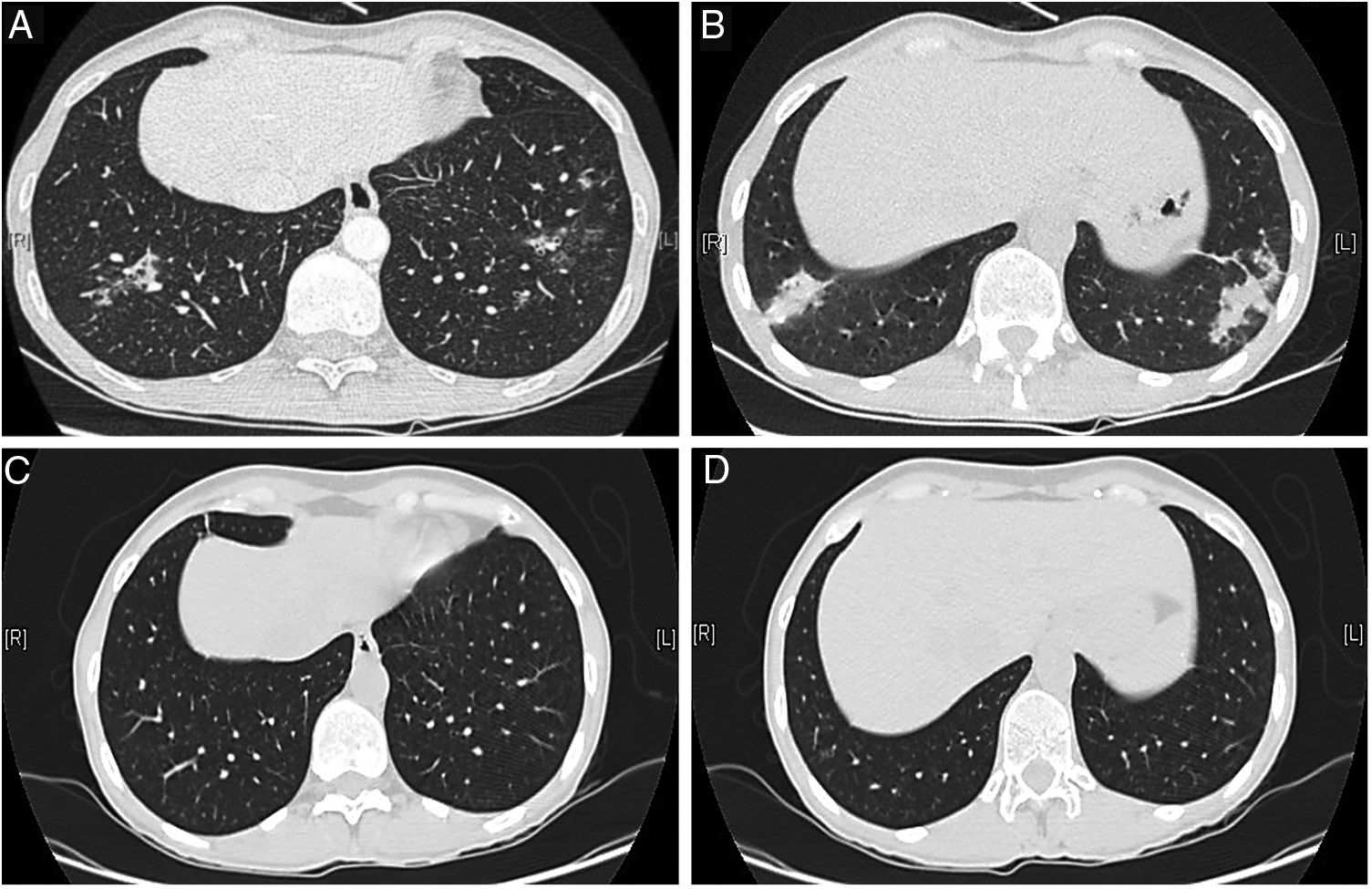
She remained in clinical remission until 4 years ago, when she noticed persistent dry cough, with periods of fever and sputum, and a rise in inflammatory markers. Sequential chest X-rays had migratory pulmonary infiltrates and computed tomography (CT) showed peribronchovascular micronodules, dilated and thick-walled bronchioles with a fluffy tree-in-bud (Fig. 1A, B). The patient was recurrently diagnosed with presumed chest infections and treated with multiple cycles of antibiotics; MTX was suspended and PDN raised to 10mg/day.
Chest computed tomography at diagnosis (A, B) and 8 months after treatment with steroids and clarithromycin (C, D). Peribronchovascular micronodules and dilated and thick-walled bronchioles with a fluffy tree-in-bud similar to “cotton-in-bud” present at diagnosis (A, B), resolved after treatment with steroids in association with 4 months of clarithromycin (C, D).
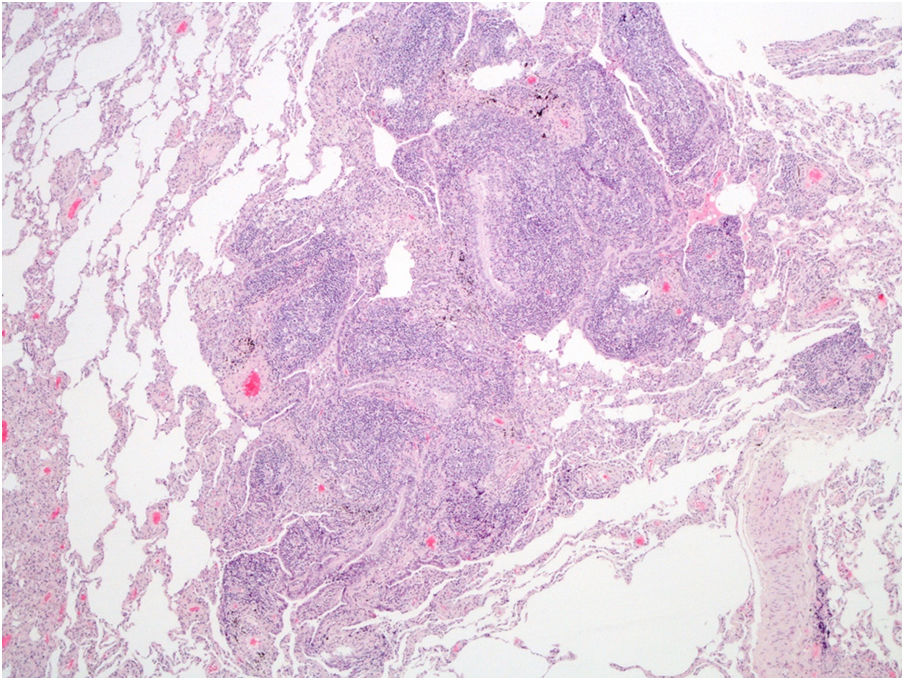
Due to symptoms’ persistence, with concomitant anorexia and loss of 10kg in 2 months, she was admitted into hospital for further evaluation. She denied smoking habits or occupational/environmental exposures. On examination she had a body mass index of 21kg/m2, pulmonary auscultation with subtle crackles in both lung bases, without arthritis, lymphadenopathies or organomegaly. Pulmonary function tests (PFTs), including carbon monoxide diffusing capacity (DLCO), were normal, without hypoxemia. Complementary exams showed negative viral serology (human immunodeficiency virus and hepatitis B/C), negative serology for acute infection by Mycoplasma pneumoniae, normal serum proteinogram, normal immunoglobulins levels and negative antinuclear antibodies. Abdominal/pelvic CT scan and endoscopy were normal. She was submitted to bronchoscopy with unspecific inflammatory changes; bronchoalveolar lavage had normal cellularity, without identification of any microorganisms, including Mycobacterium tuberculosis. Transbronchial biopsy had nonspecific lymphocytic infiltrate, forming small aggregates, without vasculitis, granulomas or neoplastic tissue. The patient was then submitted to surgical lung biopsy that demonstrated lymphoid follicles with peribronchiolar germinal centers and associated bronchiole constriction (Fig. 2), making the diagnosis of FB. The patient started PDN (0.75mg/kg/day), with clinical and radiological improvement, but one month later she developed steroid-associated neuropsychiatric symptoms and PDN was tapered. However, fever and sputum recurred, without evidence of infection. BF relapse was assumed and the addition of a second drug was discussed between rheumatologists and pulmonologists. Due to the absence of articular activity and considering the previous adverse events experienced by the patient with immunosuppressive drugs and the fear of these drugs to worsen lung disease, clarithromycin (500mg/day) was added for 4 months, with progressive symptomatic improvement and PDN tapering. Four months later complete resolution of chest CT alterations was noted (Fig. 1C, D) and PDN was stopped. The patient remains currently under no treatment for 10 months, without evidence of BF recurrence and no need for further immunosuppression.
FB was first described in 1979 by Epler et al.,2 after noticing extensive proliferation of lymphoid follicles in the bronchiolar walls of 2 patients with RA and eosinophilic fasciitis treated with d-penicillamine.
In 1985 Yousem et al.3 identified three clinicopathological groups of FB. The first two included FB secondary to connective tissue disease (CTD) and congenital/acquired immunodeficiency, respectively, and the third was considered a “primary/idiopathic” subtype.
Patients may present with persistent cough (occasionally productive), breathlessness on exertion, fever, weight loss and recurrent chest infections/sinusitis.4
Chest X-ray can be normal, but lung hyperinflation due to air trapping, small nodules, reticular, or reticulonodular infiltrates also occur.4 CT findings include centrilobular and peribronchial micronodules associated with patchy ground-glass opacities.4 Some patients also present a tree-in-bud pattern with a “cotton-in-bud” appearance.4
PFTs can show a normal, restrictive or obstructive pattern,4,5 sometimes with reduced DLCO.5 The diagnosis is histological, consisting of hyperplasic lymphoid follicles with reactive germinal centers along small airways.5
Treatment of FB usually includes steroids, mostly prednisolone 1mg/kg/day.5 Relapse when tapering steroids can occur and the addition of a steroid-sparing drug is necessary. In secondary FB, treatment is usually aimed to manage the underlying disease. The first cases of RA-related FB successfully treated with macrolides were reported in 19966 and later corroborated in a case report.7
FB is generally associated with a good prognosis, without any related deaths reported. However, younger patients with an underlying immunodeficiency tend to have more severe disease.4
In conclusion, lung involvement should be considered as a differential diagnosis in patients with CTD presenting with respiratory symptoms. FB is commonly misdiagnosed as recurrent chest infections and can be extremely challenging for the clinician. Macrolides have anti-inflammatory/immunomodulatory properties and their potential role in FB was reinforced in this work.
Conflicts of interestThe authors have no conflicts of interest to declare.
Mónica Cardoso, Department of Anatomic Pathology, Hospital Santa Maria, Centro Hospitalar Lisboa Norte.