Birt–Hogg–Dubé syndrome (BHDS) is a rare autosomal dominant hereditary disease characterized by the development of cutaneous lesions, renal tumors and pulmonary cysts.1,2 The genetic defect responsible for BHDS was mapped to a gene located on chromosome 17p11.2, which encodes a tumor suppressor protein, folliculin.1,4 Pulmonary cysts were described in the majority of patients with BHDS.1,3,4 The cutaneous findings classically described consist of the triad of hamartomas of the hair follicles (fibrofolliculomas), trichodyscomas, and skin polyps or soft fibroids (acrochordons). Renal tumors, when present, are often multiple and bilateral.3,6 BHDS is probably underdiagnosed, due to the great variability in its clinical presentation. Although several studies have shown an increased prevalence of pulmonary cysts and spontaneous pneumothorax in patients with BHDS there are few published studies exploring the tomographical aspects. The purpose of our study was to review the high resolution computed tomography scans (HRCT) of 9 patients with BHDS, analyze the most frequent tomographic patterns and their distribution in the pulmonary parenchyma, and discuss the morphological characteristics of lung cystic lesions.
We retrospectively reviewed the clinical records and chest HRCT scans of 9 patients with BHDS, six female and three males, with ages ranging from 22 to 66 years (mean of 45.6 years), randomly collected in different institutions located in three countries (Brazil, Canada and England). The diagnosis was confirmed by the association of clinical, radiological and genetic data, based on the current criteria for BHDS.5 The main diagnostic findings were presented in Table 1. The HRCT scans were independently reviewed by two observers and the discordant results were resolved by consensus.
Main diagnostic findings.
| Case, gender, age | Diagnostic findings |
|---|---|
| 1. M, 54 | MC, family history, fibrofolliculomas, pneumothorax |
| 2. F, 33 | MC, family history, pneumothorax |
| 3. M, 66 | MC, fibrofolliculomas, renal tumor |
| 4. F, 26 | MC, positive genetic tests |
| 5. M, 44 | MC, family history, renal tumor, pneumothorax |
| 6. F, 66 | MC, family history, fibrofolliculomas |
| 7. F, 48 | MC, family history, renal mixed cancer, pneumothorax |
| 8. F, 51 | MC, family history, fibrofolliculomas |
| 9. F, 22 | MC, positive genetic tests, pneumothorax |
MC, multiple cysts; M, male; F, female.
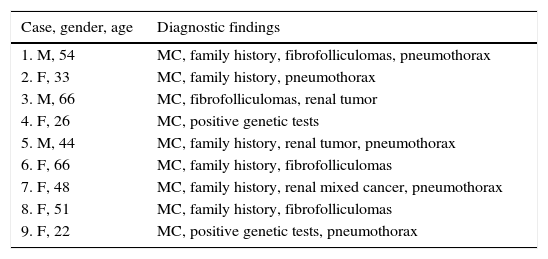
Multiple skin lesions suggestive of fibrofolliculomas were reported in four patients. Most of the lesions were described as whitish papules on the face, neck or thoracic region, and histopathological confirmation was achieved by biopsy. Two patients had a history of renal cell carcinoma, one of them with associated oncocytic cells, tumors typically found in BHDS.2,3 Two patients performed genetic tests that confirmed the presence of mutations in the folliculin gene, characteristics of BHDS, in addition to the presence of cysts, with no cutaneous or renal lesions or diagnosis of the syndrome in first-degree relatives. Six patients had relatives diagnosed with BHDS. In the case of families with cystic disease, pneumothorax or renal tumor as isolated findings, definitive diagnosis of BHDS should be performed by detecting mutation of the folliculin gene.
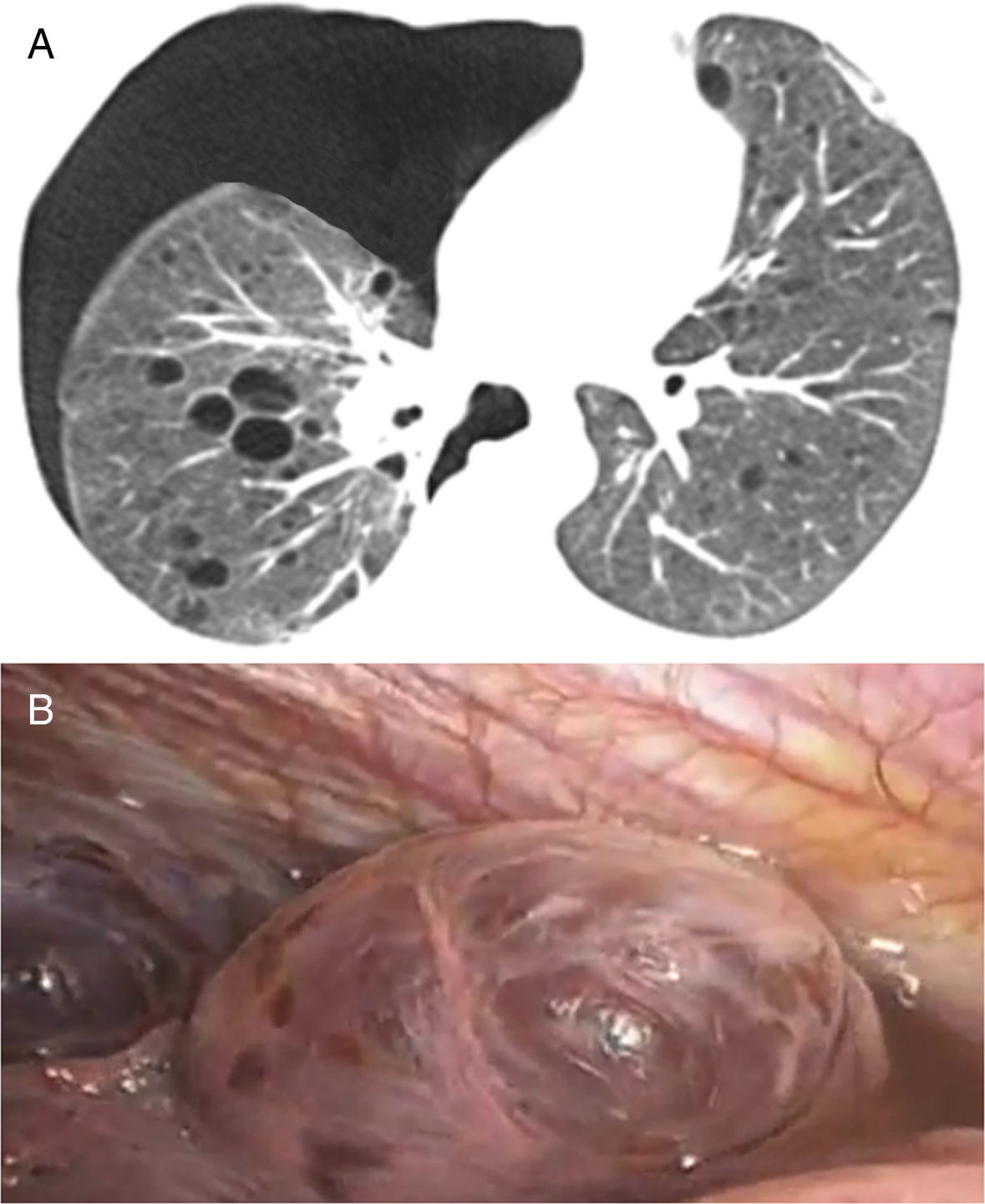
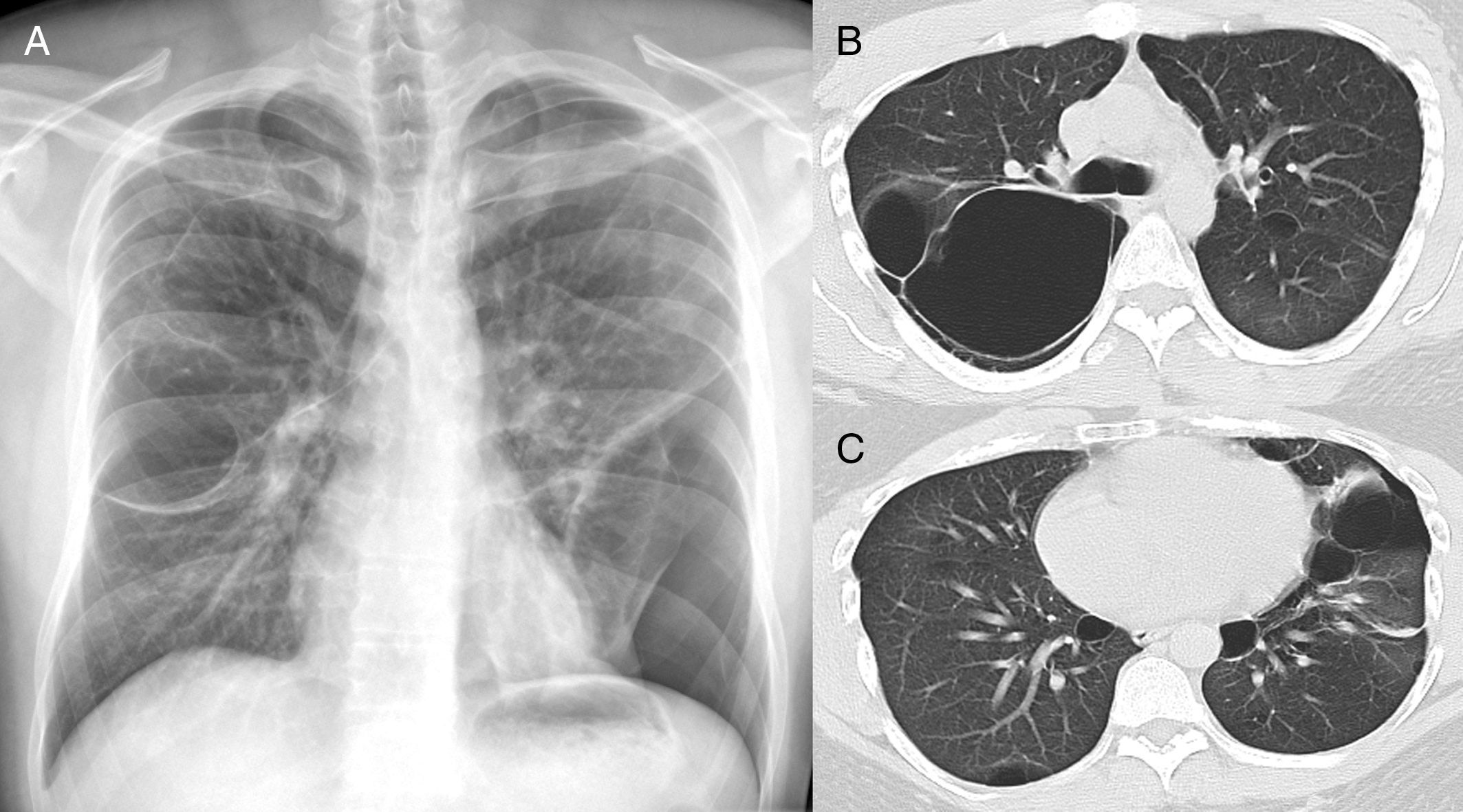
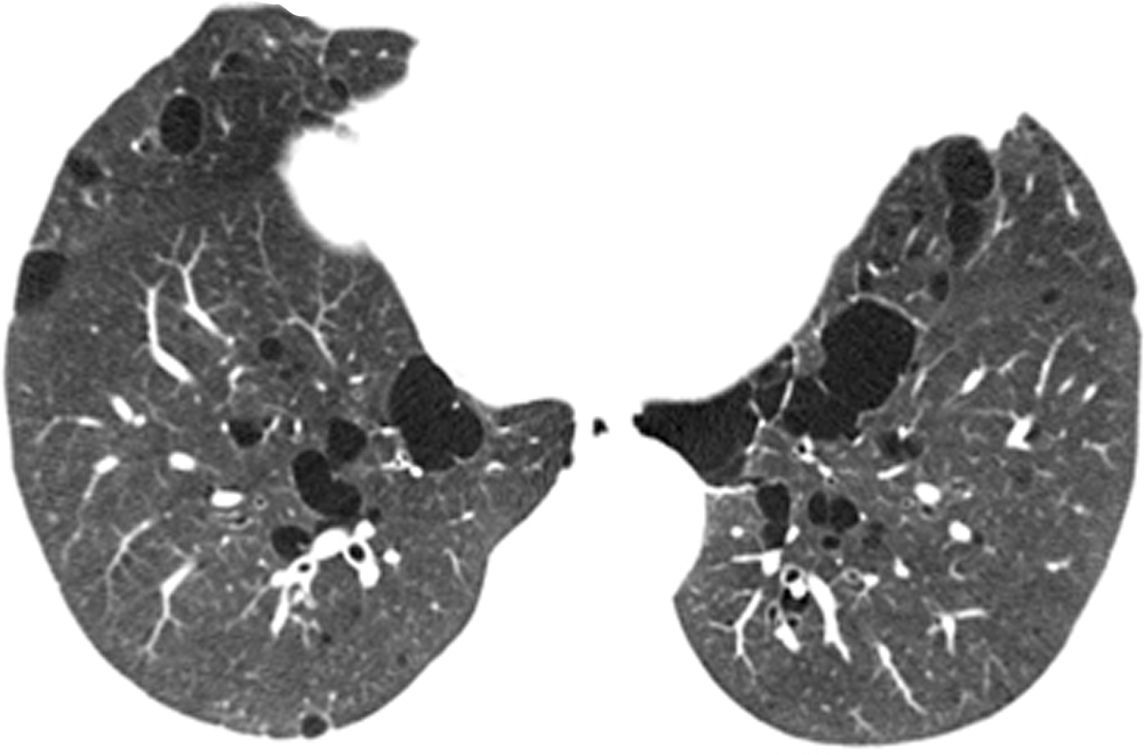
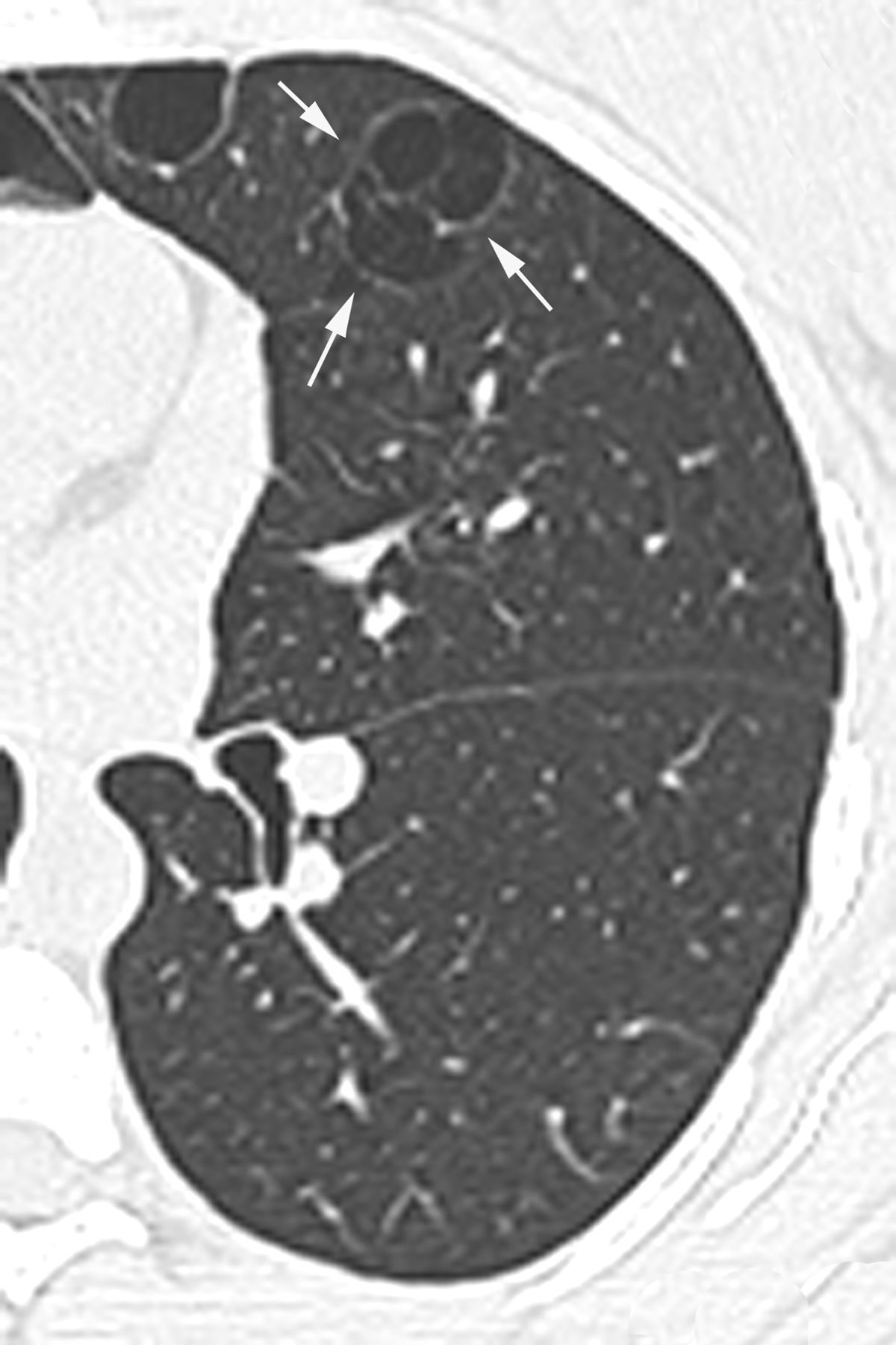
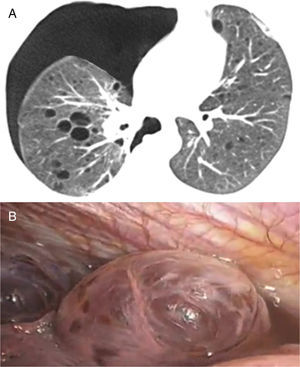
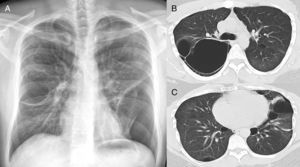
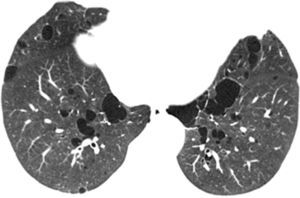
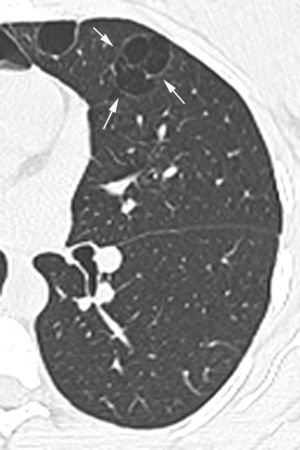
In our study chest pain was described in three patients and was related to spontaneous pneumothorax. History of spontaneous pneumothorax was present in five cases (Fig. 1). The majority of patients with BHDS and pulmonary cysts are asymptomatic. Spontaneous pneumothorax may be the only manifestation of the syndrome and although a single episode of pneumothorax may occur in these patients, recurrence has been reported in about 80% of cases.2,4 Although diffuse pulmonary cysts are a very frequent HRCT finding in individuals with BHDS, few studies focused on their HRCT presentation.6,7 Multiple bilateral pulmonary cysts were identified in all of our cases (Figs. 2 and 3). The cysts varied in number, size and shape among patients in the series, and in size and shape in each case. The lung parenchyma adjacent to the cysts did not present changes. There was predominance of cysts in the inferior zone in seven cases, no distribution preference was observed in one case, and there was superior zone prevalence also in another case. In the literature, cysts also present bilaterally, usually located in the lower lung regions.6,7 Regarding size, pulmonary cysts were classified as small (<1cm), medium (1–2cm) and large (>2cm). Six patients presented all three types, two patients had small and medium cysts and one patient had only one small and one large cyst. Four patients presented irregular cysts with internal septa as loculated or multiloculated cystic lesions (Fig. 4). The wall of the cysts was thin, less than or equal to 2mm in eight patients. The pattern of large cysts, particularly located in the lower portions of the lungs, with a multisseptated and lobulated appearance, was previously described in the literature.7 Among cystic lung diseases, lymphangioleiomyomatosis was the greatest challenge in the differential diagnosis of BHDS, especially when associated with tuberous sclerosis complex.1,6
In A, chest X-ray in postero-anterior incidence shows a round hypertransparent area in the middle third of the right lung and left pneumothorax, besides parenchymal bands in the middle third of the left lung. In B and C, high resolution computed tomography of the thorax with axial sections at the level of the bronchial bifurcation (B) and the lower lobes of the lungs (C), performed days after the pneumothorax drainage, showing pulmonary cysts and small left residual pneumothorax (arrows).
Our study presented some limitations, in that it was retrospective and patients were examined with a variety of tomographic techniques due to the different institutions involved in the study. Despite these limitations, we believe that this variation did not significantly impact on the results of the study. In conclusion, pulmonary cysts in imaging studies may be the initial finding in patients with BHDS and the careful analysis of its characteristics may suggest the presence of BHDS, highlighting the role of CT in early diagnosis. Knowledge about symptoms of BHDS by pulmonologists and radiologists is fundamental in patient care, as it leads to the early detection and management of complications, particularly renal malignancy.
Conflict of interestThe authors have no conflicts of interest to declare.