Hypersensitivity pneumonitis (HP) is an interstitial lung disease (ILD) which varies in prevalence across the world, depending on disease definition, diagnostic methods, exposure type and intensity, geographical environments, agricultural and industrial practices, and host risk factors. This study aimed to deepen knowledge about HP’s clinical characteristics, diagnosis and functional and imaging features in a cohort of HP patients from the North of Portugal. To achieve this goal, a retrospective assessment of the clinical and diagnostic data was carried out, and patients were classified and compared according to disease presentation (acute, sub-acute and chronic HP forms).
Of the 209 HP patients included (mean age 58.3 ± 16.0 years), 52.6% were female and 73.7% presented a chronic form. Most patients had prior exposure to birds (76.6%). Dyspnoea and cough were the most frequently experienced symptoms, but no statistically significant differences were found between groups (p = 0.089, p = 0.418, respectively). Fever was most common in acute HP form (p < 0.001). The most common patterns found in Chest CT were ground glass (p = 0.002) in acute/subacute presentation, and reticulation (p < 0.001) in chronic form, while mosaic attenuation, although was also frequently observed, no statistically significant differences were found between groups (p = 0.512). The most common functional pattern was restrictive (38% of patients, 73.7% with chronic HP form). Bronchoalveolar lavage lymphocytes were higher in acute and subacute forms although not reaching statistical significance (p = 0.072), with lowest CD4/CD8 ratio (p = 0.001) in acute forms.
Thus, given the significant disease heterogeneity, further studies with different populations and ambient exposures are needed to achieve a better stratification of the exposure risk, to provide proper implementation of avoidance methods and a precise diagnostic and therapeutic approach.
Hypersensitivity pneumonitis (HP), also known as extrinsic allergic alveolitis, is an interstitial lung disease (ILD) triggered by an exaggerated immune response to the inhalation of a wide variety of antigenic particles found in the environment.1,2 The most common antigens include moulds, bacteria, protozoa, animal (mostly bird) proteins and even low-molecular-weight chemical compounds. In addition, certain drugs may also cause HP, as a non-inhalational variant. It is worth mentioning that, HP may potentially arise in any work or home environment, where bacteria and moulds grow or birds are kept.2
HP prevalence varies considerably across the world, depending on the disease definition, diagnostic methods, type and intensity of exposure, geographical conditions, agricultural and industrial practices, and host risk factors. However, there is no consistent and standardized epidemiological approach to assessing the different HP forms.3 Over the years, as the disease progresses, it may lead to chronic respiratory failure, resulting from well-established pulmonary fibrosis4 or even pulmonary emphysema,5 thereby conferring a potentially serious disease status on this entity.
HP is conventionally classified into acute, subacute and chronic forms, although, to date, there are no widely accepted criteria for distinguishing the various forms.1,6,7 Moreover, there is little information on latency between exposure and symptoms onset, and it is uncertain whether they represent different stages of the disease.6
Current techniques, despite being in great demand and evolving, are not well standardized and, despite the efforts of experts to establish diagnosis based only on clinical data,8 the definitive diagnosis must be supported by additional tests (i.e., laboratorial, radiologic and histology assays), some of them of an invasive nature. In fact, disease development and clinical presentation are influenced by several factors, such as the nature and number of inhaled antigens; intensity and frequency of exposure, and even the host’s immune response, likely to be determined by a genetic background. Indeed, genetic susceptibility may explain how, after exposure, some individuals develop the disease, while others are only sensitized, but remain healthy, and others are not even sensitized.2,6
In this context, based on the above highlighted aspects, this study aims to deepen knowledge about HP’s clinical characteristics, diagnosis and functional parameters in a Portuguese cohort of HP patients. To achieve this goal, patients were classified and compared based on the disease presentation status (acute, sub-acute and chronic HP forms).
Materials and methodsStudy populationA retrospective analysis of medical records of patients diagnosed with HP followed in ILD outpatient clinic in Centro Hospitalar Universitário de São João, Porto - Portugal was performed over a period of 10 years (2007–2016). Individuals older than 18 years diagnosed with HP were included in this study, making a total of 209 patients. All patients were discussed and their diagnosis was established in the multidisciplinary team (MDT) meeting.
All relevant clinical data from the first appointment were collected, including patient demographics, medical history, smoking habits, occupational history, environmental antigen exposure history, pharmacological treatments, laboratory data, imagological features, pulmonary function data and thoracic pathology findings. This study had the approval of local Ethics Committee (Centro Hospitalar Universitário de São João, Porto – Portugal).
Diagnostic criteriaAccording to the clinical reports, exposure was classified into five categories: birds, moulds, cork, isocyanates and unknown (when the relevance of any antigen could not be established). HP diagnosis was considered in the presence of compatible exposure, clinical features (dyspnoea or cough, sputum production, asthenia, fever, weight loss, and absence of an alternative diagnosis), chest high-resolution computed tomography (HRCT) typical features, and high lymphocytic alveolitis in bronchoalveolar lavage (BAL). Patients without definitive diagnosis after the previous diagnostic approach were submitted to transbronchial lung cryobiopsy and/or surgical lung biopsy (SLB) after an individualized assessment of the indication. As not all patients underwent the same complementary tests, the results are presented according to the number of patients who were submitted to each procedure.
HP subtypes considered were acute, subacute and chronic forms. Acute form was defined as symptoms within a few hours after antigen exposure, and regression within days after removal of exposure. The subacute form was considered when progressive symptoms occurred over days or weeks. Chronic form was characterized as persistent symptoms in patients with long or persistent exposure.9 The imaging and histology data were also valuable, namely when fibrotic features were identified as their association with chronic presentation.
Thoracic high-resolution computed tomographyAll scans were obtained using a high-spatial-resolution reconstruction algorithm and, for each patient, 1- and 3-mm thick slices were obtained. Two thoracic radiologists with experience in ILD evaluation reviewed all the images for the presence or absence of abnormalities: reticular pattern, traction bronchiectasis, honeycombing, centrilobular nodules, ground glass infiltration, mosaic attenuation and emphysema.10–12 The combination of centrilobular nodules, ground-glass infiltration, mosaic attenuation, and middle and/or upper lobe distribution with or without fibrotic changes, were considered typical for HP.
Bronchoalveolar lavage fluid and flow cytometryBronchoalveolar Lavage (BAL) fluid was performed following the European Respiratory Society recommendations.13 A total of 200 mL (four aliquots of 50 mL) of sterile saline solution were instilled into the bronchial tree and gently aspirated after each instillation.
The last three recovered samples were homogenized and analysed for total cellular counts (Neubauer chamber) and viability (trypan blue exclusion) was determined. A total of 500 cells were counted on Wright-Giemsa stained cytospin slides. The samples were processed by routine flow cytometry analysis with the following combinations of monoclonal antibodies: anti-CD45-PerCP-Cy5.5 (Clone2D1), anti-CD4-FITC (Clone SK3), anti-CD8-Pe (Clone SK1), anti-CD3-APC (CloneSK7) (all BD Biosciences, San Jose, CA, USA).14 Samples were run through a BD FACS CaliburTM flow cytometer (BD Biosciences, San Jose, CA, USA) and analysed using BD CellQuest software (BD Biosciences, San Jose, CA, USA), with the acquisition of a minimum of 10,000 events. Lymphocytes were distinguished based on forward (FSC) versus side (SSC) scatters and additional gating was applied using SSC versus CD45, CD3, CD4 and CD8. All values were scored as percentages of lymphocytes.
Lung biopsiesTransbronchial cryobiopsy (TBLC) was performed using a combination of rigid (tracheoscope 14 mm, Karl Storz) and flexible (Olympus BF-XT40) bronchoscopy, under general anaesthesia with manual jet ventilation (working pressure of approximately 2 bar), using a flexible cryoprobe (90 cm with a 2.4 mm diameter; Erbokryo, Erbe, Germany). Biopsies were taken under fluoroscopic guidance from an optimal distance between the probe and the thoracic wall of 10–20 mm in different segments of the two different lobes of the same lung, usually three biopsies from lower lobe and two biopsies from the upper lobe. The probe was cooled to −85 °C with nitrogen oxide for approximately 5–6 sec.
Transthoracic biopsies were performed by a senior interventional radiologist using CT fluoroscopy. The biopsies were taken with an 18-gauge automated cutting needle after administration of local anesthesia with a subcutaneous injection of 5 mL of 2% lidocaine. In most biopsies, only one specimen was obtained, with a maximum of 2 specimens/procedure.
Surgical lung biopsy was performed on patients intubated with a double lumen endobronchial tube under general anesthesia through video-assisted thoracoscopic surgery- Endopath ETS 45 mm endoscopic linear cutter (Ethicon Endo-surgery, Cincinnati, OH- which allowed access to segments of different lobes in order to obtain multiple biopsy specimens. The biopsies sites were decided based on HRCT scan.
Biopsy samples were independently observed and evaluated by two pathologists. HP diagnosis was based on the presence of histology typical features such as centrilobular or/and perilobular fibrosis, bridging fibrosis, centrilobular fibroblastic foci, granulomas, mononuclear chronic interstitial inflammation and organizing pneumonia. If two or more findings were present, the biopsy was considered as high confidence for HP diagnosis; if only one feature was present, it was considered as low confidence.
Pulmonary function testingPulmonary function testing (PFT) was done according to a standardized protocol. Static lung volumes were measured using the plethysmography method, and the lung diffusion capacity of CO (DLCO) using the single breath-hold method.13 A restrictive ventilatory pattern was defined as forced vital capacity (FVC) <80% of the predicted value with forced expiratory volume in the first second (FEV1)/FVC ratio >80% in the absence of a static lung volume study, together with a total lung capacity (TLC) <80% of the predicted value. An obstructive ventilatory pattern was established based on FEV1/FVC ratio <70%. The concomitant presence of characteristic functional criteria for both patterns was defined as a mixed ventilatory pattern. DLCO was considered to be decreased by <75% of the predicted value.
Determination of specific IgG antibodiesThe presence of specific IgG antibodies (precipitins or enzyme-linked immunosorbent assay [ELISA]) was noted only when the relevance of a suspected exposure was uncertain. Precipitins were evaluated by Radial Immunodiffusion (RID) from Microgen Bioproducts Ltd. The test relies on the principle of double gel diffusion. When soluble antigens and homologous antibodies are placed in adjacent wells, cut into suitable diffusion media, they diffuse into each other and produce visible precipitation lines along the interface of optimal relative concentrations.
Statistical analysisCategorical variables were described as absolute values and relative frequencies and continuous variables as mean and standard deviation (SD), or minimum and maximum values, if appropriate. The relationship between categorical variables was determined using Pearson's chi-square. Comparisons among three or more groups were performed using one-way analysis of variance (ANOVA). All data were analysed using Statistical Package for the Social Sciences (SPSS, IBM Corp., USA) software, version 25.0, with alpha set at 0.05.
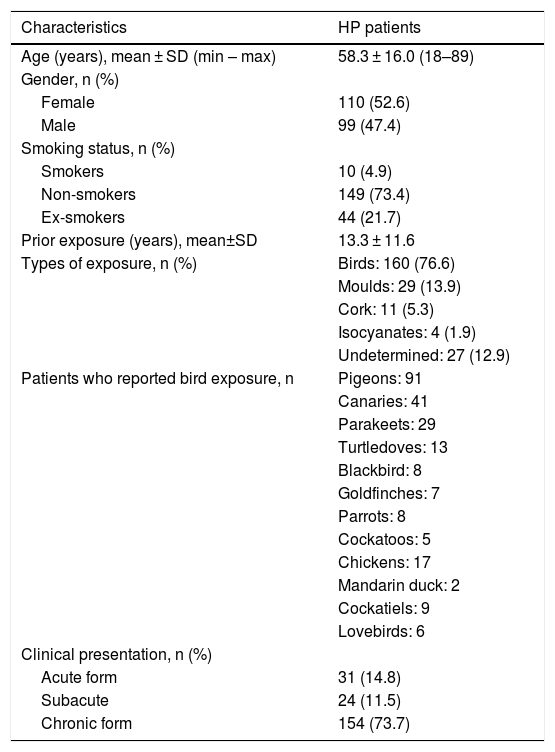
ResultsOf the 209 HP patients included, with a mean age of 58.3 ± 16.0 years, 110 (52.6%) were females and 99 (47.4%) males (Table 1). A low percentage of smokers was found (4.9%), most of the patients were (n = 149, 73.4%) non-smokers. Median prior exposure time was 10 (range: 0.2–50.0) years. Exposure apparently exclusively to birds was found in 160 (76.6%) patients, of whom 89 (55.6%) were female. The main types of birds to which patients were exposed included pigeons (n = 91), canaries (n = 41), parakeets (n = 29), turtledoves (n = 13) and cockatiels (n = 9). Among patients with bird exposure (n = 160), 57.5% were only exposed to one type of bird and 42.5% to two or more. The rest had been exposed to moulds (n = 29, 13.9%), cork (n = 11, 5.3%), isocyanates (n = 4, 1.9%), and 27 (12.9%) patients had undetermined exposure (Tables 1 and 2).
General characteristics of HP patients.
| Characteristics | HP patients |
|---|---|
| Age (years), mean ± SD (min – max) | 58.3 ± 16.0 (18–89) |
| Gender, n (%) | |
| Female | 110 (52.6) |
| Male | 99 (47.4) |
| Smoking status, n (%) | |
| Smokers | 10 (4.9) |
| Non-smokers | 149 (73.4) |
| Ex-smokers | 44 (21.7) |
| Prior exposure (years), mean±SD | 13.3 ± 11.6 |
| Types of exposure, n (%) | Birds: 160 (76.6) |
| Moulds: 29 (13.9) | |
| Cork: 11 (5.3) | |
| Isocyanates: 4 (1.9) | |
| Undetermined: 27 (12.9) | |
| Patients who reported bird exposure, n | Pigeons: 91 |
| Canaries: 41 | |
| Parakeets: 29 | |
| Turtledoves: 13 | |
| Blackbird: 8 | |
| Goldfinches: 7 | |
| Parrots: 8 | |
| Cockatoos: 5 | |
| Chickens: 17 | |
| Mandarin duck: 2 | |
| Cockatiels: 9 | |
| Lovebirds: 6 | |
| Clinical presentation, n (%) | |
| Acute form | 31 (14.8) |
| Subacute | 24 (11.5) |
| Chronic form | 154 (73.7) |
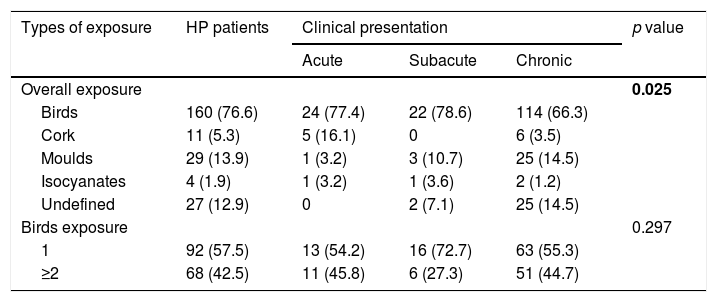
Clinical presentation based on exposure types.
| Types of exposure | HP patients | Clinical presentation | p value | ||
|---|---|---|---|---|---|
| Acute | Subacute | Chronic | |||
| Overall exposure | 0.025 | ||||
| Birds | 160 (76.6) | 24 (77.4) | 22 (78.6) | 114 (66.3) | |
| Cork | 11 (5.3) | 5 (16.1) | 0 | 6 (3.5) | |
| Moulds | 29 (13.9) | 1 (3.2) | 3 (10.7) | 25 (14.5) | |
| Isocyanates | 4 (1.9) | 1 (3.2) | 1 (3.6) | 2 (1.2) | |
| Undefined | 27 (12.9) | 0 | 2 (7.1) | 25 (14.5) | |
| Birds exposure | 0.297 | ||||
| 1 | 92 (57.5) | 13 (54.2) | 16 (72.7) | 63 (55.3) | |
| ≥2 | 68 (42.5) | 11 (45.8) | 6 (27.3) | 51 (44.7) | |
Bold: p<0.05
In terms of clinical presentation, 154 (73.7%) patients (80 females, 74 males) presented chronic HP form, of whom 114 (71.2%) had exposure to birds (Tables 1 and 2). Acute form was found in 31 (14.8%) and subacute in 24 (11.5%) patients. There is a significant association between chronic presentation and those patients with undetermined exposure (p = 0.025), while the number of birds to which patients were exposed did not have any particular association with clinical presentation (p = 0.297).
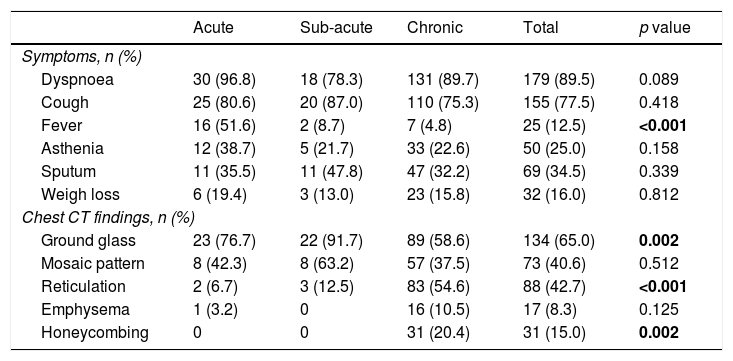
Table 3 shows the data relating to respiratory symptoms. The most frequently reported respiratory complaints were dyspnoea (n = 179, 89.5%), followed by cough (n = 155, 77.5%), sputum (n = 69, 34.5%), asthenia (n = 50, 25.0%), weight loss (n = 32, 16.0%) and fever (n = 25, 12.5%). Fever was most common among those with the acute form (p < 0.001). No significant differences were found for other symptoms.
Patients’ symptomatology and chest CT findings, according to clinical presentation.
| Acute | Sub-acute | Chronic | Total | p value | |
|---|---|---|---|---|---|
| Symptoms, n (%) | |||||
| Dyspnoea | 30 (96.8) | 18 (78.3) | 131 (89.7) | 179 (89.5) | 0.089 |
| Cough | 25 (80.6) | 20 (87.0) | 110 (75.3) | 155 (77.5) | 0.418 |
| Fever | 16 (51.6) | 2 (8.7) | 7 (4.8) | 25 (12.5) | <0.001 |
| Asthenia | 12 (38.7) | 5 (21.7) | 33 (22.6) | 50 (25.0) | 0.158 |
| Sputum | 11 (35.5) | 11 (47.8) | 47 (32.2) | 69 (34.5) | 0.339 |
| Weigh loss | 6 (19.4) | 3 (13.0) | 23 (15.8) | 32 (16.0) | 0.812 |
| Chest CT findings, n (%) | |||||
| Ground glass | 23 (76.7) | 22 (91.7) | 89 (58.6) | 134 (65.0) | 0.002 |
| Mosaic pattern | 8 (42.3) | 8 (63.2) | 57 (37.5) | 73 (40.6) | 0.512 |
| Reticulation | 2 (6.7) | 3 (12.5) | 83 (54.6) | 88 (42.7) | <0.001 |
| Emphysema | 1 (3.2) | 0 | 16 (10.5) | 17 (8.3) | 0.125 |
| Honeycombing | 0 | 0 | 31 (20.4) | 31 (15.0) | 0.002 |
Bold: p<0.05
In the chest HRCT evaluation (Table 3), besides the association between ground glass with acute/subacute presentation (p = 0.002), chronic form HP patients most frequently had reticulation (p < 0.001) and honeycombing (p = 0.002) patterns. No statistically significant differences were observed when mosaic pattern (p = 0.512) and emphysema (p = 0.125) were considered.
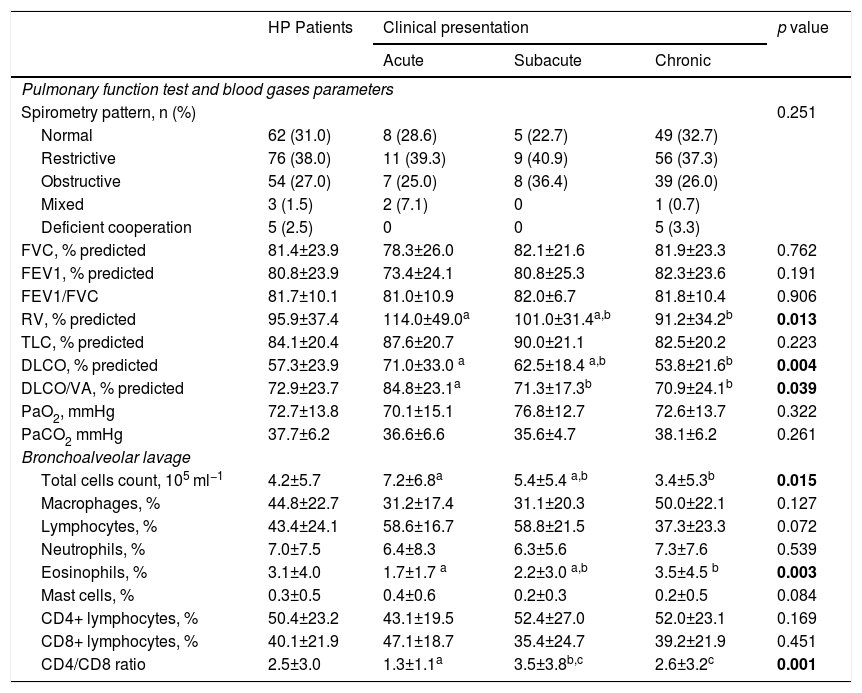
The distribution of the various ventilatory patterns, as assessed by lung function tests, is shown in Table 4. Lung Function Tests were performed on all patients with clinical condition, most of them presenting a restrictive pattern (n = 76, 38.0%), 54 patients (27.0%) showed an obstructive pattern, 3 (1.5%) a mixed pattern, and 62 (31.0%) had normal lung volumes. Five patients with a severe form of the disease failed to perform the tests properly. In general, patients with chronic HP tended to have lower RV% (p = 0.013), DLCO% (p = 0.004) and DLCO/VA% (p = 0.039) values. Regarding the blood gases parameters, PaO2 and PaCO2, no statistically significant differences were found between groups (p = 0.322 and p = 0.261, respectively).
Lung function tests, blood gases and BAL parameters in HP patients.
| HP Patients | Clinical presentation | p value | |||
|---|---|---|---|---|---|
| Acute | Subacute | Chronic | |||
| Pulmonary function test and blood gases parameters | |||||
| Spirometry pattern, n (%) | 0.251 | ||||
| Normal | 62 (31.0) | 8 (28.6) | 5 (22.7) | 49 (32.7) | |
| Restrictive | 76 (38.0) | 11 (39.3) | 9 (40.9) | 56 (37.3) | |
| Obstructive | 54 (27.0) | 7 (25.0) | 8 (36.4) | 39 (26.0) | |
| Mixed | 3 (1.5) | 2 (7.1) | 0 | 1 (0.7) | |
| Deficient cooperation | 5 (2.5) | 0 | 0 | 5 (3.3) | |
| FVC, % predicted | 81.4±23.9 | 78.3±26.0 | 82.1±21.6 | 81.9±23.3 | 0.762 |
| FEV1, % predicted | 80.8±23.9 | 73.4±24.1 | 80.8±25.3 | 82.3±23.6 | 0.191 |
| FEV1/FVC | 81.7±10.1 | 81.0±10.9 | 82.0±6.7 | 81.8±10.4 | 0.906 |
| RV, % predicted | 95.9±37.4 | 114.0±49.0a | 101.0±31.4a,b | 91.2±34.2b | 0.013 |
| TLC, % predicted | 84.1±20.4 | 87.6±20.7 | 90.0±21.1 | 82.5±20.2 | 0.223 |
| DLCO, % predicted | 57.3±23.9 | 71.0±33.0 a | 62.5±18.4 a,b | 53.8±21.6b | 0.004 |
| DLCO/VA, % predicted | 72.9±23.7 | 84.8±23.1a | 71.3±17.3b | 70.9±24.1b | 0.039 |
| PaO2, mmHg | 72.7±13.8 | 70.1±15.1 | 76.8±12.7 | 72.6±13.7 | 0.322 |
| PaCO2 mmHg | 37.7±6.2 | 36.6±6.6 | 35.6±4.7 | 38.1±6.2 | 0.261 |
| Bronchoalveolar lavage | |||||
| Total cells count, 105 ml−1 | 4.2±5.7 | 7.2±6.8a | 5.4±5.4 a,b | 3.4±5.3b | 0.015 |
| Macrophages, % | 44.8±22.7 | 31.2±17.4 | 31.1±20.3 | 50.0±22.1 | 0.127 |
| Lymphocytes, % | 43.4±24.1 | 58.6±16.7 | 58.8±21.5 | 37.3±23.3 | 0.072 |
| Neutrophils, % | 7.0±7.5 | 6.4±8.3 | 6.3±5.6 | 7.3±7.6 | 0.539 |
| Eosinophils, % | 3.1±4.0 | 1.7±1.7 a | 2.2±3.0 a,b | 3.5±4.5 b | 0.003 |
| Mast cells, % | 0.3±0.5 | 0.4±0.6 | 0.2±0.3 | 0.2±0.5 | 0.084 |
| CD4+ lymphocytes, % | 50.4±23.2 | 43.1±19.5 | 52.4±27.0 | 52.0±23.1 | 0.169 |
| CD8+ lymphocytes, % | 40.1±21.9 | 47.1±18.7 | 35.4±24.7 | 39.2±21.9 | 0.451 |
| CD4/CD8 ratio | 2.5±3.0 | 1.3±1.1a | 3.5±3.8b,c | 2.6±3.2c | 0.001 |
*different letters mean statistically significant differences between groups.
DLCO, CO Pulmonary Diffusion Capacity; FEV1, Forced Expiratory Volume in 1 s; FVC, Forced Vital Capacity; RV, Residual Volume; TLC, Total Lung Capacity; VA, Alveolar Volume.
Bold: p<0.05
Patients with chronic HP presented lower BAL total cells count (p = 0.015), while eosinophils (p = 0.003) and CD4/CD8 ratio (p = 0.001) were lower in acute HP form. Moreover, although %lymphocytes were not statistically significant (p = 0.072) between groups, there was a slightly higher percentage in the acute and subacute forms. In fact, among chronic HP patients, only 79 (61.7%) of them had %lymphocytes >30% and 39 (25.3%) did not even have lymphocytosis.
IgG antibody analysis, a marker of exposure, was only used for patients without clear exposure. Thus, of the 59 patients tested, 86.4% were negative and 13.6% (n = 8) positive for birds.
Of all patients included, 61 (29.2%) needed to perform histological analysis to obtain a clear diagnosis, of whom 33 (54.1%) needed surgical lung biopsy, 20 (32.8%) TBLC and 8 (13.1%) transthoracic biopsy. These procedures were performed only on chronic patients with overlap imaging features with other fibrotic ILDs. Thus, according to the criteria described above, of the 33 SLB, 30 (90.9%) were considered as high confidence for HP diagnosis, while in TBLC, 14 (70%) were also considered as high confidence, and with respect to transthoracic biopsy, only 1 (12.5%) met the high confidence criteria.
DiscussionSince HP results from the inhalation of an antigen to which a patient has been previously sensitized, and there is considerable discrepancy in the prevalence worldwide and limited epidemiology-related data on our population, we felt impelled to conduct this study. In this cohort, which comprises patients from the North of Portugal, a significant proportion of HP chronic presentation and a remarkable association with bird exposure were clearly evident. The high proportion of patients with chronic HP was associated with a relevant proportion of restrictive functional pattern and a significant number of patients undergoing lung biopsy, because in this form the differential diagnosis is considerably more challenging.
Although evidence has shown that the HP diagnosis has increased in recent years, there are significant geographical dissimilarities, expressed in the various ILD registries from different countries. According to these data, HP incidence is as low as 2.7% in Greece, 7.0% in Denmark or surprisingly 5.1% in Spain, and is significantly higher in other countries, such as 47.3% in India.14–17 Although we do not have data from national registries, from our experience, we believe that our situation is analogous to those countries with higher incidence of HP, and chronic HP is probably the most frequent fibrotic ILD here. In the last five years of this study (2012–2016), 634 patients were diagnosed with ILD at our centre, of which 123 (19.4%) had HP. If we remove 117 patients with sarcoidosis, which are usually included in an independent registry, HP patients account for almost a quarter of all patients, more precisely 23.7%, and were always the most frequently diagnosed ILD in all the years considered. In contrast to our findings related to gender, most of the studies found a higher proportion of men. These differences seem to be explained by the type of exposure considered and even by social, economic and cultural differences. However, it is not yet clear whether this finding really represents differences in exposures or different susceptibilities of the disease.18 Another epidemiologic aspect found in this study was the large proportion of non-smokers (73.4%), as often described in the literature.19 In fact, several reports pointed to a lower HP prevalence in smokers due to the immune impairment induced by smoking.1,6,8
On the other hand, as previously highlighted in other reports, a broad spectrum of antigens may trigger the disease, and the inciting antigen may not be identifiable in many patients with HP. Exposure to birds was the most frequently identified antigen in our setting (76.6%), which agrees with literature data.2,19 In Portugal, there are many colombophiles, and pigeon racing is one of the most popular sports. Moreover, there is a tradition of keeping pets, and birds are among the most popular. However, many other antigens may also be involved, as demonstrated in our results (moulds, cork, isocyanates), while some cases remain unknown. We believe that fungal exposure is significantly higher in our region, and the proportion of patients with this association is clearly underestimated, which is related to the difficulty in obtaining this information, since patients generally do not recognise this source, and no evaluation of the environment in their homes or workplaces has been achieved. The association between HP patients with no antigen identified with chronic forms and even with a worse prognosis has been previously described and we suggest that this is probably due to persistent exposure to the unidentified causal antigen.20,21 The hypothesis that some of these cases correspond to unidentified IPF needs to be considered, although the diagnostic accuracy achieved gives this assumption a low probability, at least in a significant number of these patients, which may be a major influence on this association.
HP clinical presentation has been classically defined in the last three decades as acute, subacute and chronic.1,6,8,9 However, there is a long controversy on this classification, namely around the subacute concept.6,7,22 In 2009, the HP study group, in an investigation involving a cohort of 165 HP patients, defined two main clusters, one had recurrent systemic symptoms and chest tightness occurring few hours after antigen exposure and chest X-rays with no relevant change in almost 30% of cases, and a second cluster with features of advanced ILD, inspiratory crackles, clubbing in one third of cases and a restrictive pattern on pulmonary function tests.22 Although these features fit in what we call acute and chronic forms, they advocate another nomenclature, especially because of the misconception these terms may suggest, as they imply that chronic HP follows acute HP, which is, at least, uncertain. One of the authors’ main conclusions is that subacute HP is particularly difficult to define.22 More recently, Vasakova et al., based on the idea that the classical classification is outdated and has no prognostic value, proposed an alternative nomenclature with two main categories based on clinical–radiologic–pathologic correlation: acute/inflammatory HP with symptoms duration of 6 months, often reversible, characterized by typical radiologic and histopathologic patterns, and chronic/fibrotic with the presence of fibrotic changes in HRCT images and/or lung tissue.7 Although this controversy is ongoing, the classic classification persists and we need more data and discussion to obtain a clear and sustainable classification in a guideline that should be the result of a consensus of leading HP experts. In our study, we decided to maintain the classic presentation, because no other alternative has been validated so far. Moreover, as this is an observational study, with no prognostic factor-related investigation, the classic classification in our opinion seems to be appropriate. In contrast to the findings of Morell et al.,19 our results showed that the most frequent form was chronic (73.7%). These findings may be due mainly to the type of antigens that are usually associated with low but persistent exposure, which is usually linked to chronic forms. A different genetic susceptibility in this specific population may also explain this high burden of chronic forms.
Following clinical evaluation, diagnosis relies on typical chest CT scan findings, high lymphocytosis in BAL, and, in some cases, histological evaluation.1,6,23 For these patients, the predominance of dyspnoea and cough was mainly found in chronic HP form and fever in the acute presentation, which fits in with established concepts.19,24 In this study, as observed in other series, the most common imaging findings were ground-glass opacification, mosaic pattern and reticulation.7,11,12,24,25 CT patterns showed acute and subacute HP forms are usually characterized by ground-glass opacities,11,25,26 and chronic form by reticulation, traction bronchiectasis and volume loss, with or without evidence of honeycombing.11,26
With BAL, lymphocytosis was most commonly documented in acute and subacute forms, like other series.1,4,6–9 Chronic forms, especially those with fibrotic features overlapping fibrotic pneumonias as common interstitial pneumonia, usually have lower or even no lymphocytosis, as we observed in this cohort where 25.3% of the patients had a normal range of lymphocytes. In addition, a trend towards a lower CD4/CD8 ratio was observed in HP patients with acute form, in contrast to subacute and chronic presentations, showing a CD4+ predominance. In fact, CD8+ lymphocyte alveolitis is frequently an acute HP feature, which decreases with exposure withdrawal, while chronic form HP patients tend to have more CD4+ than CD8+ cells.27,28
HP patients generally present restrictive ventilatory impairment29 in pulmonary function tests, although an obstructive pattern may also be present,23 which matches our findings. Most of our patients also presented a decrease in %DLCO, mainly in the chronic form. Indeed, it is clear that functional deterioration can be very serious in these patients, and may even lead to an irreversible clinical situation, in which the only therapeutic option is lung transplantation.
Although there are false positive and false negative pitfalls, it is well recognized that specific IgG antibodies against avian antigens may be useful as support evidence for HP, as a marker of exposure, but not of the disease.1,6,9 Accordingly, measurement of these IgG antibodies is only carried out in cases in which exposure to these avian antigens was not entirely clear. In addition, specific IgG antibodies may also help to establish a relationship between exposure and disease, to track possible inducers when these have not been identified or to reduce the likelihood of aetiology by feathers or fungus, if negative.1,6,9 For histological evaluation, it should be emphasized that the use of invasive complementary diagnostic tests for lung biopsy should be reserved only for cases in which the MDT evaluation, including clinical, imaging and BAL data, is not enough to conclude a definitive diagnosis. In the current series, as observed in other reports,19 a low percentage of patients required more invasive diagnostic methods. More recently, transbronchial lung cryobiopsy has enabled an accurate and less invasive diagnosis,30 certainly leading to an increase in the number of patients undergoing lung biopsy. In this cohort, despite the predominance of surgical lung biopsy (n = 33, 54.1%), these patients are mainly prior to 2014, when the cryobiopsy became available in our centre, after which almost all cases with histology in their diagnostic work-up underwent this new bronchoscopy procedure. Although 70% of TBLC was considered as high confidence in a lesser extent than the 90% within SLB, the remain 30% of TBLC considered as low confidence allowed, together with data from thoracic HRCT, to reach a confident diagnosis in MDT evaluation. The low level of confidence of transthoracic biopsy was expected, since this procedure is not considered as the most adequate in this scenario; however, all patients submitted to this intervention does not have clinical conditions for a more invasive procedure. After histological analysis, classified as high or low confidence, the final diagnosis is always established at the MDT meeting. HP is considered a difficult diagnosis, with low agreement. This was clearly demonstrated by Walsh et al. in a case-cohort study, involving 113 ILD patients evaluated by 7 MDT specialists from different regions of the world. They found a general agreement of a satisfactory Weighted-kappa coefficient (K) of 0.50 between the different MDTs, but when the different ILD diagnosis were stratified, they found in HP only a poor K of 0.25, the lowest observed.31 In fact, the chronic forms often have imaging overlaps with other fibrotic ILDs, frequently with UIP or NSIP-like features. This may also reflect the disparity in regional methodology, along with the lack of consensus guidelines for HP diagnosis. There is an urgent need for an international consensus towards a clear HP definition, diagnosis and classification.32 Our MDT is composed of 4 pulmonologists, 3 radiologists and 2 pathologists with many years of experience dedicated to ILDs and the large number of HP diagnoses discussed in this study shows at very least substantial expertise in this area.
Besides the general disagreement about HP diagnosis, specifically in chronic forms, the monocentric and retrospective design of this study is a clear limitation. The lack of antigen investigation in the patient environment clearly limits the accuracy of causal antigen recognition.
In conclusion, this study highlights the relevance of HP, particularly in chronic forms, which require a more precise and complex diagnostic assessment, due to the differential diagnosis between this form and other interstitial pneumonias. Due to chronicity and poor prognosis in a significant number of cases, there is an unmet need for meticulous research on genetic polymorphisms associated with disease predisposition and the indoor environment that may account for a significant number of cases, in order to take the first steps towards the implementation of an antigen avoidance plan.
Conflict of interestThe authors declare no conflict of interest.
Martins N. would like to thank the Portuguese Foundation for Science and Technology (FCT–Portugal) for the Strategic project ref. UID/BIM/04293/2013 and “NORTE2020—Programa Operacional Regional do Norte” (NORTE-01-0145-FEDER-000012).