Pulmonary alveolar proteinosis (PAP) is a rare pulmonary disease with specific features caused by the alveolar accumulation of surfactant, composed of proteins and lipids, due to dysfunctional pulmonary macrophages.1 It is classified into two types primary PAP and PAP secondary to leukemia, lung infections, and inhalation of mineral particles or chemical material.2 Secondary PAP (sPAP) is mainly caused by hematological disorders; sPAP associated with brucellosis is extremely rare. Here, we report a rare case of sPAP in brucellosis in a herdsman. Clinicians should consider the possibility of sPAP when chest radiography reveals abnormal findings and the bronchoalveolar lavage fluid (BALF) is milky.
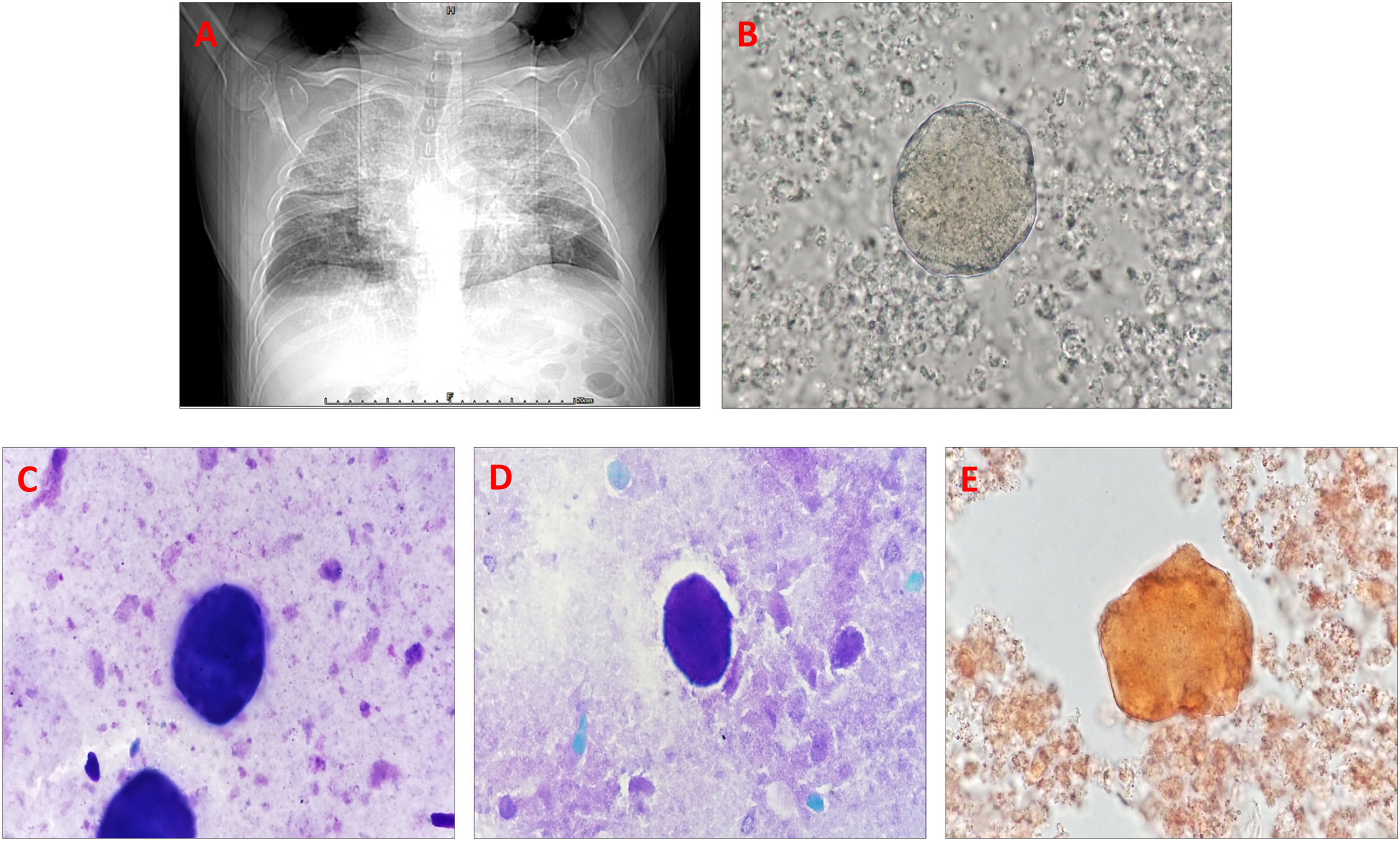
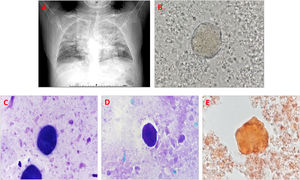
A 41-year-old male herdsman was hospitalized due to repeated cough and expectoration for 5 years, aggravated with shortness of breath for 5 months. Seven months prior, he was diagnosed with brucellosis with the presentation of lung infection at a local hospital, which improved after one month of treatment, leading to his discharge. His vital signs were normal. Physical examination and routine blood tests revealed unremarkable findings except for the clubbing of his digits. The electrocardiogram was negative, but a lung CT result revealed scattered patchy and large fuzzy shadows (crazy-paving appearance) in the bilateral lungs (Fig. 1A). Simultaneously, bronchoscopy was performed, and the BALF revealed a characteristic milky appearance. Interestingly, after centrifuging the BALF sample at 1500 rpm for 5 min and using the cell pellet to make a smear, a large number of phospholipid-rich protein aggregates were easily observed for microscopic examination (Fig. 1B). Moreover, the Wright–Giemsa staining for the above cell pellet smear showed bluish-purple staining (Fig. 1C). Also, periodic acid-Schiff (PAS) staining revealed PAS-positive proteinaceous material on the smear (Fig. 1D). Moreover, after incubating 100 uL oil red O with 1 mL of the BALF sample for 10 min and centrifuging the stained sample at 1500 rpm for 5 min, we used the cell pellet to make a smear, which clearly revealed an orange aggregate (Fig. 1E). Consequently, PAP was suspected. However, a test for serum anti-granulocyte macrophage colony-stimulating factor (GM-CSF) antibody yielded negative results; therefore, he was diagnosed with sPAP in brucellosis, and received several courses of whole lung lavage, his condition improved, and he was discharged.
Secondary pulmonary alveolar proteinosis in brucellosis in a herdsman. (A) CT reveals a crazy-paving appearance on the bilateral lungs. (B) Phospholipid-rich protein aggregates are clearly observed on the BALF smear through a direct smear for microscopic examination (1000×). (C) Wright–Giemsa staining reveals bluish-purple staining (1000×). (D) Periodic acid-Schiff-positive proteinaceous material is observed (1000×). (E) Oil red O staining reveals an orange aggregate (1000×).
Recently, abnormalities in GM-CSF signaling are implicated in the pathogenesis of autoimmune PAP, which accounts for the vast majority of cases. However, sPAP is a rarer disorder, is not dependent on GM-CSF, and mainly occurs owing to a hematological disease.3
To our knowledge, this is the first report of PAP with a recent brucellosis history and GM-CSF antibody negativity. The herdsman was diagnosed with sPAP in brucellosis. Moreover, our study suggests that if the crazy-paving appearance on CT or milky BALF are observed and characteristic globules of PAS-positive proteinaceous material are also observed on the BALF, PAP should be considered as a differential diagnosis.
Ethical considerationsWritten informed consent was obtained from this patient.
Funding informationNone.
Consent from all authorsAll authors reviewed this manuscript and agreed to submit this manuscipt.
CRediT authorship contribution statementL. Yan: Writing – original draft, Visualization. Z. Wang: Formal analysis, Writing – review & editing. J. Zhao: Formal analysis, Writing – review & editing. J. Liu: Writing – original draft, Formal analysis.