The efficiency of mucociliary transport may vary in different conditions, such as in exposure to harmful particles of the cigarette smoke. The present study evaluated the acute and short term effects of smoking on nasal mucociliary clearance in current smokers by the quantification of the Saccharin Transit Time (STT), and to investigate its correlation with the history of tobacco consumption.
MethodsNineteen current smokers (11 men, 51±16years; BMI 23±9kg/m2, 27±11 cigarettes per day, 44±25 pack-years), entering a smoking cessation intervention program, responded to a questionnaire concerning smoking history and were submitted to lung function assessment (spirometry) and the STT test. STT was assessed immediately after smoking and 8hours after smoking. The STT test was also performed in nineteen matched healthy non-smokers’ who served as control group.
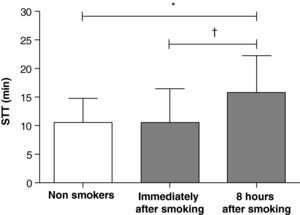
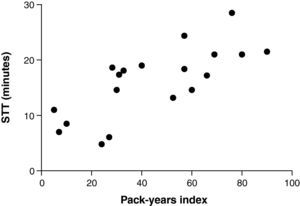
ResultsWhen compared to STT in non-smokers’ (10±4min; mean±standard deviation), smokers presented similar STT immediately after smoking (11±6min; p=0.87) and slower STT 8hours after smoking (16±6min; p=0.005 versus non-smokers’ and p=0.003 versus immediately after smoking). STT 8hours after smoking correlated positively with age (r=0.59; p=0.007), cigarettes per day (r=0.53; p=0.02) and pack-years index (r=0.74; p=0.0003).
ConclusionsIn smokers, although the mucociliary clearance immediately after smoking is similar to non-smokers’, eight hours after smoking it is reduced, and this reduction is closely related to the smoking habits.
A eficiência do transporte mucociliar pode variar em diferentes condições, como na exposição a partículas nocivas do fumo do cigarro. O presente estudo avaliou os efeitos do cigarro, tanto imediato quanto a curto prazo, no transporte mucociliar nasal de fumadores por meio da quantificação do tempo de trânsito da sacarina (TTS), e correlacionou-os com a intensidade de consumo tabágico.
MétodosDezanove fumadores ativos (11 homens; 51±16 anos; IMC 23±9kg/m2; 27±11 cigarros/dia; 44±25 anos/maço), participantes de programa de intervenção antitabagismo, responderam a um questionário referente a história tabágica e foram submetidos à avaliação da função pulmonar (espirometria) e transporte mucociliar (pelo TTS), este imediatamente e após 8 horas do acto de fumar. Para comparação, um grupo pareado composto por 19 indivíduos saudáveis não fumadores foi avaliado por meio dos mesmos testes.
ResultadosQuando comparados ao TTS de não fumadores (10±4min; média±desvio padrão), os fumadores apresentaram tempo de transporte similar imediatamente após fumar (11±6min; p=0,87) e significativamente mais lento 8 horas após fumar (16±6min; p=0,005 versus não fumadores e p=0,003 versus fumadores). Em fumadores, o TTS 8 horas após fumar correlacionou-se positivamente com a idade (r=0,59; p=0,007), o número de cigarros/dia (r=0,53; p=0,02) e o índice anos/maço (r=0,74; p=0,0003).
ConclusãoEmbora indivíduos fumadores imediatamente após fumar apresentem transporte mucociliar similar ao de indivíduos não fumadores, 8 horas após o consumo tabágico o transporte mucociliar mostra-se reduzido e relacionado com os hábitos tabágicos.
Mucociliary transport is the main defense mechanism of the respiratory tract against pathogens and toxins, both in the upper and lower airways.1,2 However, it should be noted that the efficiency of transport may vary in different conditions, such as exposure to harmful particles of cigarette smoke.3
In vitro and in vivo studies have shown that exposure of the ciliated epithelium to particles of cigarette smoke results in a significant decrease in ciliary beat frequency.4,5 Cohen et al.6 showed that ciliary beats were diminished as a result of exposure to tobacco smoke, thus impairing mucociliary clearance. These results are in contrast to the findings of Stanley et al.,7 who did not find any difference in ciliary beat frequency between smokers and nonsmokers and reported a normal ciliary beat frequency. Nevertheless, they described that mucociliary transport was slower in regular smokers, and suggested that the exposure of nasal mucosa to cigarette smoke varies considerably depending on the type of cigarette and whether the smoke is exhaled by the nose or mouth.7 Others observed, moreover, that the mucus velocity in nonsmokers is faster than in ex-smokers.8
Therefore, generally speaking, differences in mucociliary transport between smokers and nonsmokers are common. However, despite these preliminary data, mucociliary transport has not been yet studied with the necessary depth. For example, neither the differences between acute and chronic responses of the mucociliary system to tobacco smoke exposure nor the association between mucociliary transport impairment and the individual's tobacco use history have been deeply investigated. Thus, the aim of this study was to evaluate the effects of smoking on mucociliary clearance in smokers, immediately and eight hours after smoking, by quantifying the saccharin transit time (STT) and to investigate its correlation with the subject's history of tobacco consumption.
MethodsParticipantsTwo groups of subjects were evaluated: 19 current smokers, classified in their majority as heavy smokers (smoking 20 or more cigarettes/day)9 who were entering an Anti-Tobacco Awareness Program, and 19 healthy matched nonsmokers (Table 1). Individuals with cystic fibrosis, bronchiectasis, immotile cilia syndrome, a history of nasal surgery or trauma, inflammation process in the upper airway (determined during the initial interview) and smoking-related diseases certified either medically or by spirometry were excluded from the study. Non-smokers were asked if they had direct or indirect contact with tobacco smoke at home or in the work environment, to which all replied negatively. All participants were previously informed about the objectives and procedures of the study and, after signing the consent form, officially joined in the research. The study had the approval of the institution's Research Ethics Committee (Report 215/2007).
Sample Characteristics of smokers and nonsmokers (Values are mean±SD).
| Smokers (n=19) | Nonsmokers (n=19) | |
| Age (y) | 51±16 | 47±11 |
| Men/Women | 11/8 | 10/9 |
| Weight (Kg) | 70±12 | 77±17 |
| Height (cm) | 165±11* | 167±0,12 |
| BMI (Kg/m2) | 23±9 | 27±4 |
| Cigarettes per day | 27±11 | NA |
| Duration of smoking (y) | 33±11 | NA |
| Pack-years index | 44±25 | NA |
NOTE. Abbreviations: BMI, body mass index; NA, not applicable.
*P<0.05 vs healthy nonsmokers.
All individuals included in the study provided in an interview personal data and information about their smoking history (duration of smoking and number of cigarettes per day, which were used to calculate the pack-year index), and then lung function (by spirometry) and nasal mucociliary transport (by STT) were assessed. The tests were performed in a laboratory setting on two different days. On the first day, the subjects were interviewed and then underwent spirometry testing. After this, they were requested to smoke 1 full cigarette, which was immediately followed by STT quantification. All STT testing took place between 5:00pm and 7:00pm. On the following day, the same subjects were asked to start the day by maintaining their regular smoking habit but then to smoke their final cigarette between 9:00am and 11:00am, after which they were to refrain from smoking for the rest of the day. Exactly 8hours after the subject's last cigarette, i.e., between 5:00pm and 7:00pm, an STT test was performed. For confirmation purposes, all individuals were directly asked whether or not they had abstained from tobacco for the 8-hour period, to which all replied positively.
Lung Function AssessmentSimple spirometry was performed using a SpiroBank spirometer (MIR, Italy) connected to a microcomputer. The technique was in accordance with the American Thoracic Society recommendations.10 Reference values were those specified for the Brazilian population.11
Measurement of Nasal Mucociliary Clearance (Saccharin Transit Test - STT)Measurements of mucociliary transport were performed with the STT,12 as described by Rutland and Cole,13 a test known to be reproducible.14
Subjects were seated and positioned with the head slightly extended. Granulated sodium saccharin (5μg) was placed, under visual control, 2cm inside the right nostril. The time from particle placement until the first perception of a sweet taste in the mouth was recorded in minutes with a TrackPro chronometer. Individuals were instructed to maintain their initial position and were not allowed to breathe deeply, talk, cough, sneeze or sniff. They were also instructed to swallow only a few times per minute until sensing a sweet taste in the mouth. If the sensation did not occur within 60minutes, the test was stopped and the subject's ability to perceive the taste of saccharin was verified by placing it on the tongue. If the subject was able to taste the saccharin directly, the test procedures were repeated on another occasion. Each subject was clearly instructed not to use pharmacological agents such as anaesthetics, analgesics, barbiturates, tranquilizers or antidepressants for at least 12hours before the test, as well as alcohol or caffeine-based substances.
Statistical AnalysisStatistical analysis was performed with GraphPad Prism 3.0 (GraphPad Software, Inc., San Diego, USA). Normality of data distribution was verified with the Shapiro-Wilk test. Parametric statistics were used since variables were normally distributed. Results were expressed as mean±SD. Comparison between the two moments in the smoking group was performed by the paired t test. For the comparison between smokers and nonsmokers, the unpaired t test was used. Correlations were evaluated using the Pearson coefficient. The level of statistical significance was set at p<0.05 for all analysis.
ResultsThirty-eight individuals were included, nineteen smokers and nineteen nonsmokers (Table 1). No exclusions were necessary according to the exclusion criteria adopted.
Compared to STT in nonsmokers (10±4min; mean±standard deviation), smokers presented similar values immediately after smoking (11±6min; p=0.87) and slower values 8hours after smoking (16±6min; p=0.005 versus non-smokers, and p=0.003 versus immediately after smoking) (Fig. 1).
There was no significant correlation between STT immediately after smoking with any of the analysed variables. There was significant positive correlation of STT 8hours after smoking with age (r=0.59; p=0.007), consumption of cigarettes/day (r=0.53; p=0.02), duration of smoking (r=0.54; p=0.02) and pack-year index (r=0.74; p=0.0003) (Fig. 2).
DiscussionThe present study showed the acute and chronic response of nasal mucociliary clearance to tobacco smoke exposure in smokers. Immediately after exposure, mucociliary transport in smokers with relatively heavy consumption habits has values similar to those presented by non-smokers. However, the evaluation of these same smokers eight hours after smoking showed that the efficiency of the mucociliary system had decreased. This study also demonstrated that the slower the mucociliary transport eight hours after smoking, the longer the duration and intensity of the smoking habit had been.
Tobacco exposure has profound effects on mucociliary function, but the basic mechanisms have not yet been elucidated. The difficulty in explaining these mechanisms is linked to several factors: the complexity of mucociliary system components, the complexity of the various substances in cigarette smoke and the fact that the techniques for measuring time of particle removal depend not only on mucociliary velocity, but also the particle distribution and patterns of deposition.15 Additionally, the lack of standardization in the control of temperature, humidity and the moment of analysis can lead to incongruity between the results, making the comparison with studies of a similar nature rather difficult.
The STT results of smokers immediately after smoking were similar to those of nonsmokers (Fig. 1). One hypothesis for such a finding is that this apparent “increase” in nasal mucociliary transport immediately after smoking, an acute epithelial response, could represent a defense against an aggressor agent such as cigarette smoke. It may have been due to an increase in ciliary beat frequency because of stimulation to the inflammatory mediators,16 or it could have been the result of stimulation to the nerve receptors found around the luminal cells.17 In a study by Lindberg & Dolata,18 the acute exposure of rabbits to cigarette smoke was associated with an increase in mucociliary activity. This effect was primarily mediated by a reflex from stimulation of NK1 receptors, followed by the irritant effects of smoking on sensory afferent nerves of the upper airway.
The difference between the STT immediately and 8hours after smoking may also be an effect of nicotine on the autonomic nervous system (ANS). It should be emphasized that this substance causes neural sympathetic stimulation, which leads to activation of the body's general metabolism.19 Moreover its effect on the parasympathetic nervous system is related to nicotinic acetylcholine receptors, which increase in situations of chronic smoking.20 Such conditions could alter mucociliary transport, since the nose has motor, sensory and autonomous innervation.21 The stimulated ANS generates nasal effects such as glandular hypersecretion and vasodilatation,22 which could justify increased mucociliary transport. Thus, the normal STT values found in smokers immediately after smoking may be related to the activation effect triggered by the sympathetic nervous system, which could include a possible acceleration of cilia beating. However, the circulating nicotine is metabolized in two hours, indicating that after this period the stimulatory effect ceases, and the smoker's cilia beat rate (or the efficiency of transport and defense mechanism) returns to its “normal” (i.e., impaired), such as was observed in this study after the abstinence period.
The present study suggests that, in a sample composed of individuals without immediate exposure to pollutants, there was slow mucociliary clearance in chronic smokers eight hours after smoking compared to healthy nonsmokers (Fig. 1). Stanley et al.7 compared the time of mucociliary transport in smokers and nonsmokers, and also concluded that the smokers’ time (21±9min) was greater than that of nonsmokers (11±4min). However, no differences were detected in mean ciliary beat frequency. If such slowness of mucociliary activity is not associated with changes in cilia beat, it may be a consequence of structural changes, such as a reduced number of cilia and/or changes in mucus viscoelasticity.7 Using clinical data and radiographic and respiratory function tests, Verra et al.23 observed that the percentage of structural abnormalities in the bronchial epithelium was higher in smokers and former smokers than in the control group. The authors suggested that chronic smoking can induce an increase in the number of abnormal cilia, which could play a role in the impairment of tracheobronchial clearance.23 Moreover, the fact that the group of ex-smokers also exhibited structural abnormalities shows that tobacco abstinence was insufficient to restore the already damaged structures.
The exposure of the nasal mucosa to cigarette toxins depends on the number and type of cigarettes smoked and smoking habits.7 This study observed a significant correlation between STT values after 8hours without smoking and the subjects’ consumption of cigarettes per day, duration of smoking and pack-year index. Possibly, the effect of chronic exposure to tobacco caused more intense damage in the population included in this study, since cigarette consumption was high and long-lasting. The fact that STT results immediately after smoking were not related to pack-year index reminds us that the action of nicotine on the ANS remains unchanged, even with the possible anatomical and physiological changes resulting from chronic exposure to cigarette smoke.
Finally, it should be pointed out that the findings of this study add new information to the scarce literature on mucociliary transport in smokers, particularly the acute response of this respiratory defense mechanism to tobacco smoke and the relationship of mucociliary transport with smoking habits. However, studies that include longer periods of abstinence and smokers with a wider range of smoking habits should be carried out using different protocols in order to verify the extent of damage to mucociliary transport in smokers.
ConclusionsIn conclusion, although the mucociliary clearance immediately after smoking is similar to nonsmokers, eight hours after smoking it is reduced. This reduction is related to the intensity of tobacco consumption, characterizing a deficiency of this pulmonary defense mechanism in this population.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Please cite this article as: Proença M, et al. Efeito imediato e a curto prazo do cigarro sobre o transporte mucociliar nasal de fumadores. Rev Port Pneumol. 2011; 17: 172–176.