H7N9 infection has raised serious concerns worldwide. Pregnant women were considered to be at a high risk of influenza infection. Normal pregnancy was dependent on T helper (Th) 2 deviation. However, whether pregnancy influences the immune status of influenza H7N9 patients has not been reported.
Case reportHere, we reported a case of pregnant woman in the first trimester with H7N9 infection compared with the two non-pregnant female H7N9 patients for clinical features and relevant immunological changes. We found that there were no differences in plasma levels of Th1 and Th2 cytokines between the pregnant and non-pregnant patients, and there was no Th2 deviation in the acute phase. However, the Th2 deviation was recurrent along with the clearance of infection in the H7N9 pregnant patient.
ConclusionThese cases highlighted that the pregnant patient infected with H7N9 could induce an effective Th1 immune response equal to that of non-pregnant patients with H7N9 virus infection, although the pregnancy itself could lead to a Th2 deviation. These data suggested that pregnant patients could acquire a similar antiviral response for H7N9 infection versus non-pregnant patients.
The outbreak of H7N9 human infection in March 2013 has raised serious concern worldwide. H7N9 infection was characterized by a rapidly progressive pneumonia, respiratory failure, acute respiratory distress syndrome (ARDS) and fatal outcomes, with high rates of mortality and morbidity.1 Cases occurred in a first wave (n=133) from February to May 2013. Reports of human infection decreased during the summer, and have increased since October, demonstrating a second wave, most likely in conjunction with cooler temperatures.2 Updated to 21 February 2014, more than 200 new cases were reported in 2014.3 In the first wave of H7N9 infection, two patients (1.5%) were pregnant (one in the first trimester and the other in the second trimester).4
A series of studies have reported that pregnant women were considered to be at a high risk of influenza infection and influenza-related complications.5–7 The physiological changes and the “immune deviation” occurring during pregnancy were thought to explain this vulnerability.8 Normal pregnancy was considered to be dependent upon T helper (Th) 2 deviation—a decreased production of the Th1 cytokine (IFN-γ) and an increased production of the Th2 cytokines (IL-4 and IL-6), especially an increased ratio of Th2/Th1.9–11
However, the detailed immunological mechanism remains unclear. Further understanding of the relevant immunological changes in pregnant H7N9 patients is necessary to guide interventions and prevent severe lung diseases. The occurrence of H7N9 infection in a pregnant patient offered a unique opportunity to study the pathological and immunological process.
Case presentationIn April 2013, a 36-year-old pregnant female (case 1) was admitted to Negative Pressure Wards at the First Affiliated Hospital, School of Medicine, Zhejiang University, because of fatigue, fever and cough. The patient was in the ninth week of pregnancy (G2P1LC1). Initial symptoms (vomit and fatigue) began 7 days before admission. During the next two days, the high fever (39.5°C) and cough developed along with sputum. Other symptoms, such as sore throat, rhinorrhoea, conjunctivitis, haemoptysis, dyspnoea, diarrhoea, abdominal pain, myalgia, skin rash and respiratory failure, were absent. She was admitted to a local district hospital 3 day before admission. Laboratory examinations elicited the following results: white blood cells (WBC) 5.9×109/L, neutrophils 71.9%, C-reactive protein 5.8mg/L, hemoglobin 144g/L, platelet count 124×109/L, blood pH 7.446, partial pressure of carbon dioxide 31.0mmHg, partial pressure of oxygen 67.3mmHg (the normal ranges in Table S1). Though Tylenol and supportive treatment were given, the high fever persisted. H7N9 infection was laboratory-confirmed 7 days after symptoms onset, and then the patient was transferred to our hospital. The APACHE-II score was 7 at admission, and 75mg oral oseltamivir twice daily were given. Epidemiological and clinical features of the patient were presented in Table 1.
Clinical features of pregnant and non-pregnant H7N9 patients.
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (years) | 36 | 37 | 33 |
| Sex | Female | Female | Female |
| Place of residence | Zhejiang, China | Jiangxi, China | Zhejiang, China |
| Contact history with poultry | Yes | No | No |
| Underlying medical disorders | Early pregnancy | No | No |
| Chronic smoker | No | No | No |
| Presumed incubation period (days)a | 10 | Uncertain | Uncertain |
| Presenting symptoms | |||
| Temperature (°C) | 39.5 | 39.2 | 39.3 |
| Sore throat | –b | – | – |
| Rhinorrhoea | – | – | – |
| Conjunctivitis | – | – | – |
| Cough | +c | + | + |
| Sputum | + | + | + |
| Haemoptysis | – | – | – |
| Dyspnoea | + | + | + |
| Nausea or vomiting | + | – | + |
| Diarrhoea | – | – | – |
| Abdominal pain | – | – | – |
| Myalgia | – | – | + |
| Fatigue | + | – | + |
| Skin rash | – | – | – |
| Respiratory failure | – | – | – |
| APACHE-II score | 7 | 6 | 8 |
| Time between onset of symptoms and initiation of oseltamivir (days) | 7 | 7 | 7 |
| Antibiotics given | No | No | No |
| Glucocorticoid given | No | No | No |
| Immunoglobulin given | No | No | No |
| Days in hospital | 13 | 11 | 12 |
APACHE-II score, acute physiology and chronic health evaluation II score.
During the same period, two non-pregnant female patients (case 2 and case 3, 33 and 37 years, respectively) were infected with H7N9 and hospitalized. They were comparable in gender, age, underlying medical disorders and disease condition upon hospital admission (Table 1) with the pregnant patient. The two patients were admitted 7 days after symptom outset. The APACHE-II score of the two patients were 6 and 8 at admission, respectively. Thus, they were matched as controls.
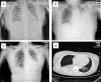
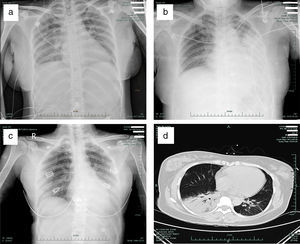
The primary abnormality of all patients on the chest radiograph and CT was patchy or diffuse consolidation in single or multiple lobes. The degree of lung lesions in the pregnant patient was comparable with that in the non-pregnant patients on admission (Fig. 1a–d). Clinical symptoms were improved in all three patients after oseltamivir administration for 6 days, and the hospital days were 13, 11 and 12, respectively (Table 1).
Representative radiographic findings in the pregnant and non-pregnant H7N9 patients. (a) Chest radiograph of case 1 (pregnant) taken on admission, showing bilateral pulmonar patchy high density shadows; (b) Chest radiograph of case 2 taken on admission, showing a patchy, high density shadow in the middle-inferior lobe of the left lung; (c) Chest radiograph of case 3 taken on admission, showing a patchy, high density shadow of the inferior lobe of the left lung; (d) CT scan of case 1 (pregnant) taken on admission, showing significant consolidation of the inferior lobe of the right lung and a patchy, high density area in the inferior lobe of the left lung.
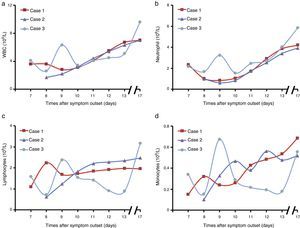
During the hospitalization of the three patients, the levels of peripheral blood cells including WBC, neutrophil, lymphocytes and monocytes were measured using a standard clinical automatic analyzer. The counts of WBC and neutrophil showed a decrease in the early stage of disease, and then recovered to the normal range on the 11th day (Fig. 2a and b). On the 8th or 9th day after symptom outset, WBC counts reached the lowest levels in the three patients (2.8×109/L, 1.7×109/L and 2.6×109/L, respectively). Notably, there was no lymphopenia in the pregnant patient, while lymphopenia was noted in the non-pregnant patients (Fig. 2c and Table S2). Meanwhile, monocyte counts were low in the early stage of disease, and then increased in the three patients with disease progression (Fig. 2d and Table S2).
Blood samples were available for immunological assessment. The classification of the peripheral blood mononuclear cells (PBMCs) was determined by flow cytometry on the Beckman coulter FC 500 MPL (Beckman Coulter, CA, United States) and the percentage of positive stained cells was analyzed using MXP software (Beckman Coulter, CA, United States). The percentages of T lymphocyte subsets and NK cells in peripheral blood cells from the three patients were in a normal range during disease progression (Table S2). The levels of iNKT cells were similar in the three patients (Table S2). However, the percentages of B cells (CD19+) in PBMCs were lower in the pregnant patient.
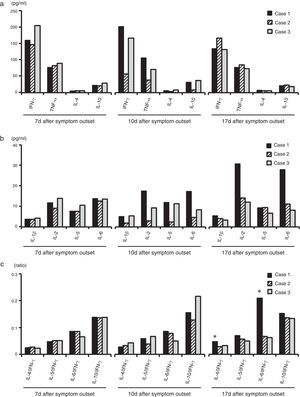
H7N9 virus infection was known to induce substantial cytokine activation.12,13 Thus, plasma levels of 8 cytokines were measured in the acute phase (on the 7th and 10th day after symptom outset) and the recovery phase (on the 17th day after symptom outset) by the Bio-Plex Pro Human Cytokine 27-Plex Immunoassay kit (Bio-Rad, Hercules, CA) in a Luminex analyzer (Luminex Corporation, Austin, TX) according to the manufacturer's protocol. The levels of these cytokines were higher in the three patients than those in healthy women (Fig. 3 and Table S3). There were no differences in plasma levels of IFN-γ, TNF-α, IL-4 and IL-10 between the pregnant and non-pregnant patients on the 7th, 10th and 17th day (Fig. 3a). Meanwhile, plasma levels of IL-1β, IL-2, IL-5 and IL-6 were similar in the three patients on the 7th day (Fig. 3b). Notably, the levels of IL-2 and IL-6 were almost twice as high in the pregnant woman as those in the non-pregnant patients on the 10th and 17th day (Fig. 3b). Moreover, the ratios of Th2/Th1 cytokines such as IL-4/IFN-γ, IL-5/IFN-γ, IL-6/IFN-γ and IL-10/IFN-γ were similar in the three patients on the 7th day, and were comparable with healthy women (Fig. 3c). The ratio of IL-4/IFN-γ in the pregnant patient was at a normal level, but lower than that in the non-pregnant patients on the 10th day. Importantly, on the 17th day, the ratio of IL-4/IFN-γ was high in the pregnant patient versus non-pregnant patients as well as healthy women (Fig. 3c). In addition, the ratio of IL-6/IFN-γ was high in the pregnant patient versus non-pregnant patients and healthy women on the 17th day (Fig. 3c). These results suggested no Th2 deviation in the acute phase, but the Th2 deviation recurred along with the clearance of infection in the H7N9 pregnant patient.
Plasma cytokines levels in the pregnant and non-pregnant H7N9 patients. (a) Plasma levels of IFN-γ, TNF-α, IL-4 and IL-10 in the pregnant and non-pregnant H7N9 patients; (b) Plasma levels of IL-1β, IL-2, IL-5 and IL-6 in the pregnant and non-pregnant H7N9 patients; (c) The ratios of plasma IL-4/IFN-γ, IL-5/IFN-γ, IL-6/IFN-γ and IL-10/IFN-γ in the pregnant and non-pregnant H7N9 patients.
Recently, H7N9 influenza viruses have caused sporadic infections in humans. To the best of our knowledge, only one patient with pregnancy has been reported,14 but the details of the immunological process in this patient have not been published.
Pregnant women were considered to be at increased risk for influenza virus infection and influenza-associated death in previous influenza A virus pandemics.5–7 The physiological changes and the “immune deviation” occurring during pregnancy were thought to explain this vulnerability.8 Normal pregnancy can be considered a Th2-dependent process.9 Meanwhile, progesterone has been shown to significantly inhibit the expression of IFN-γ in PBMCs in pregnant cows.15 In pregnant animals, infection with influenza H1N1 induced further Th2 deviation due to a further significant increase in the levels of IL-4 than IFN-γ on the basis of the already skewed Th2 immunity.16 Moreover, there were lower peripheral blood T lymphocytes, higher levels of proinflammatory cytokines, and worse fetal development occurred in pregnant mice than non-pregnant controls.17 However, vaccination with an inactivated influenza vaccine could effectively protect pregnant women from H1N1 infection, which had no correlation with the Th1/Th2 status in pregnant women.18
In our study, we found that there were no differences in plasma levels of Th1 and Th2 cytokines between the pregnant and non-pregnant patients, and there was no Th2 deviation in acute phase. However, the Th2 deviation recurred along with the clearance of infection in the H7N9 pregnant patient.
In conclusion, these cases highlighted that the pregnant patient infected with H7N9 could induce an effective Th1 immune response equal to that of non-pregnant patients with H7N9 virus infection, although the pregnancy itself could lead to a Th2 deviation. These data suggested that pregnant patients could acquire a similar antiviral response for H7N9 infection versus non-pregnant patients.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by the National Natural Science Foundation of China (81271810), Important National Science and Technology Project-Research of Clinical treatment for Emerging severe acute respiratory infectious disease (2014ZX10004006), the Science and Technology Department Foundation of Zhejiang Province (2014C03039), Doctoral Fund of Ministry of Education of China (20120101110009).