The real life effectiveness, safety and the use of omalizumab for Portuguese patients with uncontrolled persistent allergic asthma are not sufficiently well known. The objective of this report was to make an evaluation, in a post-marketing, non-interventional, observational registry, of the Portuguese population included in the eXpeRience study.
MethodsThe methods used in this report are the same as the global eXpeRience ones, applied to a Portuguese sub-population. Patients with uncontrolled allergic asthma who had started omalizumab within the previous 15 weeks were enrolled and received omalizumab add-on therapy for 24 months. The physicians’ global evaluation of treatment effectiveness (GETE), asthma symptoms and control (ACT score), quality of life (mini-AQLQ score), exacerbations, and serious adverse events (SAE) were reported.
ResultsOf the 943 patients recruited in the eXpeRience registry, 62 patients were from Portugal. 62.1% of them were observed to be responders with good/excellent GETE assessment at Week 16. Clinically meaningful improvements in asthma control (ACT score) and quality of life (mini-AQLQ score) were observed with omalizumab therapy at Months 12 (mean change: +7.7 [n=35]; +2.1 [n=20], respectively) and 24 (mean change: +7.0 [n=26]; +2.7 [n=13], respectively). Asthma symptoms and rescue medication usage were reduced to ≤1 day/week at Month 24 from a baseline of ≥3.5 days/week. The proportion of patients with no clinically significant exacerbations increased from 6.5% during pre-treatment (n=62) to 50% at Month 12 (n=54) and 60% at Month 24 (n=45).
ConclusionThe findings from the Portugal subpopulation of eXpeRience registry confirm that omalizumab add-on therapy is efficacious and well tolerated in the management of uncontrolled persistent allergic asthma. Another pertinent issue is the fact that the Portuguese subpopulation response is similar to the international population average of the study.
Asthma is a chronic disease affecting approximately 300 million people worldwide and it is estimated that this number will have increased by 100 million by 2025.1 In Portugal, a national asthma survey showed a prevalence of current asthma in 6.8% of the population.2
Although clinical trials with combinations of inhaled corticosteroids (ICS) and β2-agonists have shown good asthma control in most patients,3 10–15% have severe forms of the disease, which remain uncontrolled or poorly controlled despite receiving optimized therapies. These patients are associated with high frequency of asthma symptoms and exacerbations, impaired lung function, and poor quality of life.4
Omalizumab is a recombinant humanized monoclonal anti-immunoglobulin E (IgE) that is used in Europe for add-on therapy with CSs and long-acting β2-agonists (LABAs) for patients aged ≥6 years with severe persistent allergic asthma.5 Clinical trials suggest that omalizumab add-on therapy reduces asthma exacerbations, decreases oral corticosteroid (OCS) and emergency room (ER) visits as well as improves symptom severity and quality of life.6
This report focuses on the outcomes of omalizumab in combination with current therapy in Portuguese patients enrolled in a 2-year, global, post marketing registry named eXpeRience.7
MethodsThe eXpeRience registry was a multicenter, international, single-arm, open-label, observational study that collected data from patients receiving omalizumab for uncontrolled persistent allergic asthma, in Europe, Canada, and Asia.7
Clinical symptoms, asthma control, lung function measures, obtained forced expiratory volume in 1s [FEV1], peak expiratory flow [PEF]), exacerbations, concomitant medication, and status of comorbidities were collected at 16 weeks and at 8, 12, 18, and 24 months after initiation. Response to treatment was evaluated at week 16 by the physician's Global Evaluation of Treatment Effectiveness (GETE) assessment 8, physical examination, and review of patient notes. Patients were rated on a 5-point scale: 1-excellent (complete control of asthma), 2-good (marked improvement), 3-moderate (discernible, but limited improvement), 4-poor (no appreciable change), and 5-worsening.8 Patients with an “excellent” or “good” response were considered responders; those with “moderate”, “poor” and “worsening” response were considered non-responders. Asthma control was assessed through Asthma Control Test™ (ACT™), which is calculated from patients’ responses to five questions concerning interference with activities, shortness of breath, nocturnal symptoms, rescue medication, and self-rating of asthma control.9 The patient's ACT™ score range from 5 (poorly controlled) to 25 (well-controlled). The mini-Asthma related Quality of Life Questionnaire (mini-AQLQ) assessment was used for evaluating the functional impairment in patients.10 Other assessments included symptoms, use of rescue medication, lung function, asthma-related healthcare resources utilization, days of missed work, and OCS/ICS use. The annualized data on healthcare resources utilization and exacerbations at Month 12 and 24 were analyzed; 1-year history of these parameters was used for comparison. Serious adverse events (SAEs) were reported, regardless of suspected causality, within 24h of the event's occurrence. Primary effectiveness analysis was performed on the intent-to-treat (ITT) population, which comprised all enrolled patients.
ResultsThe eXpeRience study enrolled 943 patients and 62 were recruited from Portugal. Demographic and clinical characteristics of these patients are presented in Table 1. Overall, 15 patients discontinued the registry (24.2%): 4 were lost to follow-up, 1 withdrew consent, and 10 discontinued for other reasons. At the end of registry, from 55 patients with available data, omalizumab treatment was continued in 41 (66.1%).
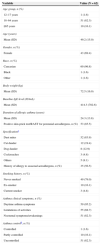
Baseline demographics and clinical characteristics.
| Variable | Value (N=62) |
|---|---|
| Age group, n (%) | |
| 12–17 years | 1 (1.6) |
| 18–64 years | 51 (82.3) |
| ≥65 years | 10 (16.1) |
| Age (years) | |
| Mean (SD) | 49.2 (15.0) |
| Gender, n (%) | |
| Female | 43 (69.4) |
| Race, n (%) | |
| Caucasian | 60 (96.8) |
| Black | 1 (1.6) |
| Other | 1 (1.6) |
| Body weight (kg) | |
| Mean (SD) | 72.5 (16.9) |
| Baseline IgE level (IU/mL) | |
| Mean (SD) | 414.3 (782.6) |
| Duration of allergic asthma (years) | |
| Mean (SD) | 24.3 (13.8) |
| Positive skin-prick test/RAST for perennial aeroallergens, n (%) | 53 (85.5) |
| Specificationa | |
| Dust mites | 52 (83.9) |
| Cat dander | 12 (19.4) |
| Dog dander | 8 (12.9) |
| Cockroaches | 3 (4.8) |
| Others | 5 (8.1) |
| History of allergy to seasonal aeroallergens, n (%) | 35 (56.5) |
| Smoking history, n (%) | |
| Never smoked | 49 (79.0) |
| Ex-smoker | 10 (16.1) |
| Current smoker | 3 (4.8) |
| Asthma clinical symptoms, n (%) | |
| Daytime asthma symptoms | 59 (95.2) |
| Limitations of activities | 55 (88.7) |
| Nocturnal symptoms/awakenings | 51 (82.3) |
| Asthma controlb, n (%) | |
| Controlled | 1 (1.6) |
| Partly controlled | 10 (16.1) |
| Uncontrolled | 51 (82.3) |
IgE, immunoglobulin E; RAST, radioallergosorbent test; SD, standard deviation.
Maintenance therapy at baseline included OCS in 17.7% patients with mean total daily dose of 16.67mg (prednisolone equivalent), and rescue medication in 91.9% patients in the week before.
The individual last time point (the time point at which the patient was last recorded as being on treatment) in majority of patients was Month 24 (72.6%) while corresponding patients’ percentages at other time-points were 1.6% (baseline), 1.6% (Week-16), 9.7% (Month-8), 8.1% (Month-12), and 6.5% (Month-18).
Physician's global evaluation of treatment effectivenessFrom patients with GETE assessment at Week 16 (n=29), 62.1% were responders (excellent/good in 10.3% and 51.7%, respectively), and 37.9% were non-responders. One patient at Week 16 was identified as non-responder and stopped treatment. From patients with GETE data at any time-point (n=62), 61.3% were responders (excellent/good in 8.1% and 53.2%, respectively), 32.3% non-responders, and 6.5% had no GETE assessment.
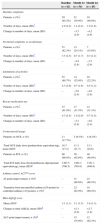
Asthma symptoms and rescue medication useOmalizumab reduced patients’ symptoms, activity limitations, and rescue medication use in the week before Month 12. Moreover, symptoms or rescue medication use at ≥3.5 days/week at baseline, reduced to ≤1 day/week at Month 24 (Table 2).
Effect on asthma symptoms, rescue medication use, asthma control, and quality of life (ITT population).
| Baseline (n=62) | Month 12 (n=54) | Month 24 (n=45) | |
|---|---|---|---|
| Daytime symptoms | |||
| Patients, n (%)* | 59 (95.2%) | 21 (38.9%) | 18 (40.0%) |
| Number of days, mean (SD)* | 4.9 (2.2) | 1.4 (2.4) | 1.0 (1.8) |
| Change in number of days, mean (SD) | – | −3.3 (2.9) | −4.2 (2.6) |
| Nocturnal symptoms or awakenings | |||
| Patients, n (%)* | 51 (82.3%) | 13 (24.1%) | 7 (15.6%) |
| Number of days, mean (SD)* | 3.5 (2.5) | 0.7 (1.7) | 0.3 (1.2) |
| Change in number of days, mean (SD) | – | −2.9 (2.8) | −3.5 (2.6) |
| Limitations of activities | |||
| Patients, n (%)* | 55 (88.7%) | 14 (25.9%) | 10 (22.2%) |
| Number of days, mean (SD)* | 4.3 (2.6) | 0.7 (1.6) | 0.5 (1.2) |
| Change in number of days, mean (SD) | – | −3.6 (2.9) | −4.0 (2.8) |
| Rescue medication use | |||
| Patients, n (%)* | 57 (91.9%) | 17 (31.5%) | 14 (31.1%) |
| Number of days, mean (SD)* | 4.3 (2.4) | 1.0 (2.0) | 0.7 (1.4) |
| Change in number of days, mean (SD) | – | −3.3 (2.9) | −4.0 (2.4) |
| Corticosteroid usage | |||
| Patients on OCS, n (%) | 11 (17.7%) | 5 (9.3%) | 4 (8.2%) |
| Total OCS daily dose (prednisolone equivalent mg), mean (SD)c | 16.7 (17.2) | 11.3 (21.7) | 13.1 (24.6) |
| Patients on ICS, n (%) | 60 (96.8%) | 50 (92.6%) | 40 (88.9%) |
| Total ICS daily dose (beclomethasone dipropionate equivalent μg), mean (SD)d | 1497.5 (766.5) | 1494.3 (765.0) | 1351.1 (846.8) |
| Asthma control, ACT™ score | |||
| ≥2 point improvement, n (%)a | – | 31 (88.6%) | 23 (88.5%) |
| Transition from uncontrolled asthma (≤19 points) to controlled asthma (>19 points), n (%)a | – | 22 (62.9%) | 18 (69.2%) |
| Mini-AQLQ score | |||
| Mean (SD)e | 3.1 (1.1) | 5.1 (1.5) | 5.8 (1.3) |
| Change in mini-AQLQ, mean (SD)b | – | +2.1 (2.0) | +2.7 (1.8) |
| ≥0.5 point improvement, n (%)b | – | 15 (75.0%) | 12 (92.3%) |
SD, standard deviation.
Mean FEV1% predicted improved by 8.6% (18.4%) at Month 12 (n=27) and 9.6% (16.6%) at Month 24 (n=27). PEF improved from baseline by mean of 22.0 (82.5)L/min at Month 12 (n=28) and 45.4 (66.7)L/min at Month 24 (n=23).
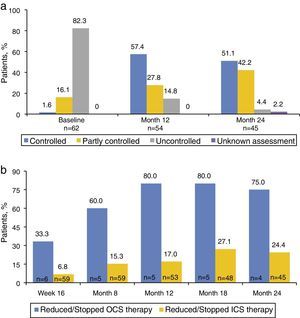
Asthma control and quality of lifeA higher percentage of patients had controlled or partly controlled asthma in the week before Months 12 and 24 than reported at baseline (Fig. 1a). Omalizumab treatment improved asthma control as measured by the mean ACT™ score at all time points, with improvements versus baseline (n=45) by 7.7 (5.1) at Month 12 (n=35) and 7.0 (4.1) at Month 24 (n=26). Over 60% of the patients were observed to transition from uncontrolled asthma at baseline to controlled asthma, and over 80% of patients showed improvements in ACT™ score by ≥2 points at Month 12 and 24. Similarly, quality of life with respect to the mini-AQLQ score and proportion of patients with clinically improvements in mini-AQLQ score at Months 12 and 24 compared with baseline were improved (Table 2).
ExacerbationsThe mean numbers of clinically significant and severe exacerbations reduced at Months 12 and 24 compared with baseline. Moreover, the number of patients with no clinically significant and no severe clinically significant exacerbations increased at Months 12 and 24 (66.1%, 20.4% and 11.1% of patients with severe exacerbations at baseline, Months 12 and 24, respectively).
Healthcare utilization useAsthma-related medical healthcare use and missed days of work due to asthma at Months 12 and 24 were significantly reduced (Table 2).
Corticosteroid useAt baseline, higher numbers of patients were on OCS/ICS maintenance treatments; this proportion reduced at Month 12 and 24 along with a corresponding increase in patients who showed reduction or discontinuation of OCS/ICS use (Fig. 1b). Of the patients on OCS/ICS maintenance treatments, the mean total daily dose went down over time from baseline to Month 24.
Serious adverse eventsOverall, two SAEs were reported in two patients. The tracheobronchitis incidence was not suspected to be study-related, whereas pulmonary embolism was suspected to be study-related leading to treatment withdrawal.
DiscussionIn Portugal, asthmatic patients are managed under Portuguese Guidelines on control of asthma adapted from GINA guidelines.5 Both reports recommend adjunctive therapy with anti-IgE such as omalizumab, for patients whose symptoms are not controlled despite combination therapy including high doses of ICSs and LABAs. This report evaluated efficacy and safety of omalizumab treatment in Portuguese patients with uncontrolled asthma enrolled in the eXpeRience registry.7
The majority of patients in Portugal (62.1%) were rated as excellent/good responders. This finding is consistent with the global registry, where 69.9% of patients were responders.7
Portuguese patients treated with omalizumab reduced daytime/nocturnal symptoms, activity limitations and rescue medication usage. Furthermore, there were reductions in the clinically significant and severe clinically significant exacerbations similar to those observed in the global registry and other real-life studies in Portugal.7,11
Clinically meaningful improvements in important outcomes (ACT™ and mini-AQLQ) together with over 90% of patients showing ≥0.5 score increase at Month 24 were also registered. These findings are comparable with another Portuguese study where there has been progress for ACT™.12 Moreover, the 8.6% and 9.6% improvements in the FEV1% predicted at Months 12 and 24 observed in this analysis are similar to those observed in another Portuguese study with omalizumab.13
In this study, the number of patients on OCS maintenance treatment reduced from 11 to 4 patients at Month 24 accompanied with a dose reduction from 16.7mg (prednisolone equivalent) to 13.1mg.
After omalizumab treatment, asthma-related healthcare resource utilization and days of missed work due to asthma were reduced, according to the global registry.7 Efficacy outcomes were consistent with this study confirming omalizumab efficacy in treatment of uncontrolled severe allergic asthma.6 In Portugal the percentage of patients with SAEs (3.2%) was lower when compared with the eXpeRience registry (6.9%).7
The major limitation of this study is the low number of patients, along with other limitations of the overall eXpeRience registry discussed previously.7 A remarkable issue is that the results observed in Portugal were comparable with the observations from the eXpeRience population. Hence, the benefits of the omalizumab therapy observed in Portugal could be further supported by the global study that included comparatively larger patient numbers.7
In conclusion, Portuguese results are consistent with the global eXpeRience registry and are supportive of the existing evidence that omalizumab therapy improves lung function, reduces asthma symptoms, exacerbations, healthcare utilization, and improves ACT™ score and quality of life in these patients.
Ethical disclosuresProtection of human and animal subjects’The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestManuel Pereira Barbosa has participated as consultant in advisory boards and has been involved in clinical trials sponsored by Novartis.
Antonio Bugalho de Almeida has participated as consultant in advisory boards and as speaker in meetings organized by Boehringer Ingelheim, GlaxoSmithKline, Novartis Farma and Pfizer. Antonio Bugalho de Almeida has also been involved in clinical trials sponsored by Boehringer Ingelheim and Novartis Farma.
Catarina Pereira, Chien-Wei Chen, Panayiotis P. Georgiou, Guy Peachey are employees of Novartis.
The eXpeRience study group is also composed of the following investigators: Luis Taborda and La Salete Valente, Centro Hospitalar da Cova da Beira; Graça Castel Branco and João Fonseca, Hospital de São João, José Ferreira and Aurora Carvalho, Centro Hospitalar de Vila Nova de Gaia/Espinho, Ulisses Brito, Hospital Central de Faro, Gil Duarte and Margarida Trindade, Centro Hospitalar Lisboa Norte, Hospital Pulido Valente, Fernando Nogueira, Centro Hospitalar de Lisboa Ocidental, Fernando Rodrigues, Hospital Fernando Fonseca, Joaquim Saraiva Cruz, Hospital de Santarém, António Domingos, Centro Hospitalar de Torres Vedras, Pedro da Costa and Paulo Santana, Unidade Local de Saúde do Norte Alentejano, Luis Ferreira, Hospital de Sousa Martins, Luisa Melão, Hospital do Espírito Santo, Laura Simão, Centro Hospitalar do Tâmega e Sousa, Jorge Roldão Vieira, Hospital Garcia de Orta, Jorge Pires, Ana Arrobas and Carlos Mendonça, Centro Hospitalar de Coimbra. The authors would like to thank Swathi Malla, Novartis, for providing the medical writing support in the development of this report. The writing support was funded by Novartis Farma – Produtos, Portugal. The registry was designed and sponsored by Novartis Pharma AG.