Adverse drug reactions (ADR) to first-line antituberculosis drugs are frequent and have important implications that may affect the effectiveness of treatment and course of tuberculosis (TB).
Material and methodsRetrospective data analysis of clinical records and national registration forms from patients with ADR to first line antituberculosis that occurred between 2004 and 2013 at a Portuguese Pulmonology Diagnostic Centre, and from a case–control population matched by sex, age and year of initiation of treatment.
ResultsOf the 764 patients treated with antituberculosis drugs, 55 (52.7% male, 92.7% European, mean age 50.8±19.5 years) had at least one severe ADR and six had a second ADR, for a total of 61 events. The most frequent ADR were hepatotoxicity (86.9%), rash (8.2%) and others, such as ocular toxicity, gastrointestinal intolerance and angioedema (4.9%). Isoniazid, alone or in combination, was the antituberculosis drug most associated to toxicity. Due to ADR, treatment time changed an average of 1.0±2.6 months (range −3.4 to 10.6). There was no correlation between age or gender and the overall incidence of ADR although we found a significant association between younger age and an increased risk of hepatotoxicity (P=0.035). There was also a statistically significant relationship between ADR and diabetes mellitus (P=0.042) but not for other comorbidities or multi-resistant TB risk factors.
ConclusionsThis study found a high frequency of ADR with strong impact on subsequent therapeutic orientation. What seems to be of particular interest is the relationship between ADR and diabetes mellitus and the increased frequency of hepatotoxicity in younger patients.
The global burden of tuberculosis (TB) is currently still very high. It represents one of the major causes of infectious disease mortality despite the availability of curative treatment.1 Moreover, latent tuberculosis infection (LTBI) affects over a third of the worldwide population posing a high risk for later reactivation of the disease. Therefore, TB chemotherapy is essential as an intention-to-cure measure and also as a public health policy to prevent Mycobacterium tuberculosis transmission.
Portugal remains the only country in Western Europe with an intermediary-prevalence of tuberculosis.1 In 2012 alone, a total of 2399 new cases of TB were reported, for an estimated 22.8 cases per 100,000 inhabitants, while 157 patients died of TB while on treatment.2
TB disease is treated with multiple effective drugs combined for a long period of time that extends for a minimum of 6 months, but in special circumstances there is demand for longer treatments.3,4 Treatment completion, defined by the number of doses taken over a period of time, is vital to prevent treatment failure, relapse and development of resistance.
Four drugs form the core of treatment regimens for drug-susceptible organisms: PZA, isoniazid (INH), rifampicin (RIF) and ethambutol (EMB). All of these first-line drugs can induce mild to severe adverse drug reactions (ADR), including: hepatotoxicity associated to INH, RIF and PZA; cutaneous reactions associated to INH, PZA and EMB; gastrointestinal intolerance to RIF; and retrobulbar neuritis related to EMB.5–7 Since streptomycin (SM) was no longer considered a first-line drug there was a diminished incidence of ototoxicity, its main side effect.8 Regarding latent tuberculosis infection (LTBI), standard regimens as, for example, short-courses of INH plus RIF are associated with an increased risk for ADR such as hepatotoxicity.7,9,10
When an ADR occurs, especially when severe, one or more drugs may have to be discontinued or treatment interrupted, which has multiple implications, particularly extended treatment time and an increased risk for drug resistance, treatment failure and relapse. In addition, use of alternative regimens are also related to important side effects and pose compliance issues. All patients must be closely monitored to immediately recognize major ADR and activate proper therapeutic measures.
Material and methodsPatient selection and follow-upBetween 2004 and 2013, all patients who experienced major ADR due to first-line antituberculosis drugs provided on ambulatory basis by our Pulmonology Diagnostic Centre (PDC) were identified and all demographic and clinical data were retrospectively collected. Data from a group control consisting of a matched population by sex, age and year of initiation of treatment were also assembled for comorbid conditions analysis.
Inclusion criteria were as follows: age of 17 years or older; established diagnosis of active TB, either pulmonary or extrapulmonary, or proven LTBI; treatment regimen with first-line antituberculosis drugs; confirmed ADR to at least one of the antituberculosis drugs.
TB was defined as a clinically compatible illness confirmed by microbiological analysis or, when negative, a case with compatible clinical, radiologic, and/or histologic findings, and exhibiting positive response to treatment. LTBI was defined by the absence of clinical features suggestive of TB, normal chest radiographic examination and a positive tuberculin skin test (TST) induration. Since 2008, after the acquisition of laboratory means to perform the interferon gamma release assay (IGRA) using the ELISA method (Quantiferon-TB® Gold In-tube), this highly specific test was implemented as an active screening routine to confirm all positive TST reactions from patients suspected of having TB infection and support treatment decision.
Once having started antituberculosis treatment, all patients were closely monitored for surveillance of possible ADR and other disease-related complications. The first appointment occurred 15 days after beginning treatment, the second appointment at 1-month of treatment and the following ensued at monthly intervals.
Blood tests including complete blood count (CBC), liver transaminases, bilirubin (total and partial) and creatinine, were routinely checked at 15, 30 and 60 days of treatment and, afterwards, once each 2 months until the treatment was completed. Viral serology for hepatitis and HIV were routinely assessed, mostly before starting antituberculosis treatment.
Whenever necessary, as in the event of a serious side effect or complication, the patient was consulted or serum analyses were done beyond the usual routine. Also, if required, other complementary studies were added to the standard evaluation.
From patient's clinical records and national registration forms, information was collected regarding sex, age, nationality, residency, career occupation, symptoms, risk factors such as intravenous drug abuse, alcoholism or residence in a social support community, comorbidities, site of disease, diagnostic tests, initial treatment, adverse effects and outcomes.
Major ADR were defined as those that required discontinuation of one or more drugs, shift to second-line drugs or hospital admission. When all drugs were suspended, they were restarted after clinical and/or laboratory complete resolution, in a sequential way to possibly isolate the drug that caused the toxicity. A second-line treatment was initiated when a slow resolution was anticipated or treatment interruption was not desirable.
Hepatitis was defined as liver transaminases more than three times higher than the upper limit of normal in the presence of symptoms such as nausea, vomiting or abdominal pain, or transaminases more than five times the upper limit of normal without symptoms.
Significant episodes of cutaneous toxicity related to extensive eruption, symptoms like fever and mucous membrane involvement, or to sustained complaints while on medication with anti-histaminic drugs.
Gastrointestinal disturbance was considered major when there was no improvement despite measures such as combining the timing of administration to main meals or proton pump inhibitor medication.
Loss of visual acuity was routinely assessed by a careful ophthalmologic examination and related to anti-TB drugs when all other causes had been excluded.
Statistical analysisData analysis was executed by IBM SPSS® for Windows version 20.0. All demographic and clinical features were reported using frequency and descriptive analyses. Qualitative data were evaluated by chi-square test and quantitative data by Student's t test.
ResultsDemographicsDemographic and clinical characteristics of the population are described in Table 1. From a total of 764 patients treated with antituberculosis regimens, 55 experienced at least one major ADR (7.2%). Six patients experienced a second ADR, for a total of 61 advents. The majority were male (52.7%) with a mean age of 50.8 years (range 17 to 94 years). Most of them were European (92.7%) and a small cluster belonged to Portuguese-spoken language African countries (7.3%), such as Cape Verde, Mozambique and Guinea Bissau.
Demographic and clinical characteristics of patients with severe adverse drug reactions to antituberculosis drugs: 2004–2012.
| n (%) | |
|---|---|
| Age, years | |
| Mean (range) | 50.8±19.5 (17.0 to 94.0) |
| 17–34 | 11 (20.0) |
| 35–59 | 26 (47.3) |
| 60–94 | 18 (32.7) |
| Sex | |
| Male | 29 (52.7) |
| Female | 26 (47.3) |
| Continent of origin | |
| Europe | 51 (92.7) |
| Africa | 4 (7.3) |
| Method of detection | |
| Active (screening of contacts) | 11 (20.0) |
| Active (screening of other groups) | 6 (10.9) |
| Passive (symptoms) | 38 (69.1) |
| Year of diagnosis | |
| 2004–2008 | 24 (43.6) |
| 2009–2013 | 31 (56.4) |
| Tuberculosis | |
| Active disease | 40 (72.7) |
| Pulmonary | 30 (54.5) |
| Lymphatic | 5 (9.1) |
| Pleural | 3 (5.5) |
| Genitourinary | 1 (1.8) |
| Cutaneous | 1 (1.8) |
| Latent infection | 15 (27.3) |
| Radiographic pattern | |
| Cavitated | 13 (23.6) |
| Non-cavitated | 20 (36.4) |
| Normal | 22 (40.0) |
| Comorbidities | |
| No comorbidities | 42 (76.4) |
| Neoplasm | 4 (7.3) |
| HIV positive | 3 (5.5) |
| Arterial hypertension | 3 (5.5) |
| Liver disease | 2 (3.6) |
| Silicosis | 1 (1.8) |
| Obstructive pulmonary disease | 1 (1.8) |
| Chronic renal insufficiency | 1 (1.8) |
| Psychiatry disease | 1 (1.8) |
| Risk factors | |
| Community resident | 5 (9.1) |
| IV drugs | 3 (5.5) |
| Alcohol abuse | 5 (9.1) |
| TB diagnostic test | |
| Microbiological analysis | 28 (50.9) |
| Smear positive/culture negative | 17 (30.9) |
| Smear negative/culture positive | 8 (14.5) |
| Smear and culture positive | 3 (5.5) |
| Tissue sample biopsy | 12 (21.8) |
| IGRA test | 15 (27.3) |
Fourteen patients (25.5%) had at least one important comorbidity. The most frequent was malignant disease, followed by HIV and hepatitis C co-infection.
Five patients were currently living in community centres, two of which for treatment of drug addiction. One other patient was also a drug addict and five patients were alcoholic.
Tuberculosis diagnosisOf all 55 patients with severe ADR, 40 (72.7%) had been diagnosed with active TB, either pulmonary or extrapulmonary, while the remaining 15 (27.3%) had confirmed LTBI. Pulmonary TB was the most frequent form of the disease, accounting for more than half of the patients. Extrapulmonary TB was diagnosed in 10 patients: 5 ganglionic, 3 pleural and 1 each of genitourinary and cutaneous TB.
TB was detected mainly through passive screening of symptomatic patients (69.1%). Those remaining were diagnosed by active screening (30.9%), either by the investigation of contacts or assessment of other groups, such as patients submitted to anti-tumour necrosis factor (TNF)-alpha treatment.
All patients with LTBI or extra-pulmonary TB had normal chest radiographs. Pulmonary cavity TB was recognized in 13 patients while other radiographic patterns occurred in the remaining 17 patients with pulmonary TB and also in all patients with pleural TB.
Pulmonary TB was diagnosed mainly by smear microscopy and/or culture analysis from respiratory sample specimens (93.5%). Positive microbiological test results were obtained from sputum samples in 17 of these patients and from bronchial lavage samples in the other 10 patients. The remaining three patients with pulmonary TB had negative microbiological tests and were diagnosed through histological analysis from lung biopsies. Genitourinary TB was diagnosed by urine culture examination. All other cases of extrapulmonary TB (pleural, lymphatic and cutaneous) were diagnosed by specimen biopsy from the corresponding tissue sample. All 15 cases of LITB were confirmed by positive IGRA tests.
Treatment regimenThe most common initial treatment, prescribed to 24 patients, was the combination of four first-line anti-TB drugs: INH, RIF, PZA and EMB. One patient was treated with the same regimen except that SM replaced EMB. Nine patients with pulmonary cavity lesions were included in the four-drug standard treatment with EMB because of SM in-hospital absence. Fifteen patients initiated treatment with a three-drug regimen that included INH, RIF and PZA. EMB was not integrated in this group once drug susceptibility was already known and M. tuberculosis was fully susceptible.
The chosen therapeutic regimen for all patients with LTBI included in our study was the combination of INH and RIF administered on a daily basis during a 3-month period. However, the best therapeutic approach was determined on an individual basis and the patient characteristics. Other options included 9 months of INH or 4 months of RIF.
Whenever possible, fixed-doses combination tablets of rifater (INH, RIF and PZA) and rifinah (INH and RIF) were prescribed on a daily continuous basis. However, the emergence of an ADR frequently meant suspension of the combination treatment to remove or assess the ADR-related antituberculosis drug.
Only two patients with pulmonary TB did not undergo directly observed therapy (DOT), which was strictly related to accessibility difficulties. Neither of the patients with genitourinary and cutaneous TB and none of the patients with LTBI were treated with DOT strategy. Ultimately, a total of 36 patients (65.5%), 28 with pulmonary TB, all 5 patients with lymphatic TB and all 3 patients with pleural TB, were submitted to DOT.
The majority of patients who suffered severe ADR experienced a deviation from the total predicted time of treatment with a mean variation of 1.0±2.6 months (range −3.4 to 10.6) due to treatment suspension or termination (Tables 2 and 3).
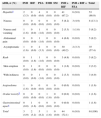
Adverse drug reactions to first-line antituberculosis treatment, related symptoms and correspondence to specific antibacilar drugs.
| ADR, n (%) | INH | RIF | PZA | EMB | SM | INH+RIF | INH+RIF+PZA | Total |
|---|---|---|---|---|---|---|---|---|
| Hepatitisa | 2 (3.3) | 0 (0.0) | 4 (6.6) | 0 (0.0) | 0 (0.0) | 41 (67.2) | 6 (9.8) | 53 (86.9) |
| Nausea | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (8.2) | 3 (4.9) | 8 (13.1) |
| Nausea and vomiting | 1 (1.6) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 2 (3.3) | 1 (1.6) | 5 (8.2) |
| Abdominal pain | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 4 (6.6) | 0 (0.0) | 5 (8.2) |
| Asymptomatic | 1 (1.6) | 0 (0.0) | 2 (3.3) | 0 (0.0) | 0 (0.0) | 30 (49.2) | 2 (3.3) | 35 (57.4) |
| Rash | 0 (0.0) | 1 (1.6) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 3 (4.9) | 0 (0.0) | 5 (8.2) |
| Skin eruption | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 2 (3.2) |
| With itchiness | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 2 (3.3) | 0 (0.0) | 3 (4.9) |
| Angioedema | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) |
| Ocular toxicityb | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) |
| Gastrointestinal upsetc | 0 (0.0) | 1 (1.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.6) |
| Total | 3 (4.9) | 2 (3.2) | 5 (8.2) | 1 (1.6) | 0 (0.0) | 44 (72.1) | 6 (9.8) | 61(100) |
INH: isoniazid; RIF: rifampicin; PZA: pyrazinamide; EMB: ethambutol; SM: streptomycin.
Antituberculosis treatment regimens, association between treatment onset and adverse drug reactions, and effect on total treatment timea.
| n (%) | |
|---|---|
| Initial first-line antituberculosis treatment | |
| INH+RIF+PZA+EMB | 24 (43.6) |
| INH+RIF+PZA | 15 (27.3) |
| INH+RIF | 15 (27.3) |
| INH+RIF+PZA+SM | 1 (1.8) |
| Adverse reaction onset, months after treatment initiation | |
| ≤2 | 19 (31.1) |
| >2 and ≤3 | 15 (24.6) |
| >3 and ≤6 | 13 (21.3) |
| >6 | 14 (23.0) |
| Association between antituberculosis drug and adverse reaction | |
| Confirmed | 11 (18.0) |
| Presumed | 50 (82.0) |
| Total treatment time, months | |
| Mean, range | 7.4±3.6 (0.2 to 19.3) |
| 0–3 | 11 (20.0) |
| 4–6 | 8 (14.5) |
| 7–9 | 16 (29.1) |
| 10–12 | 17 (30.9) |
| >12 | 3 (5.5) |
| Variation of treatment time compared to predicted, months | |
| Mean (range) | 1.0±2.7 (−3.4 to 10.6) |
| Increased | 24 (43.6) |
| Decreased | 19 (34.5) |
| No variation | 12 (21.8) |
INH: isoniazid; RIF: rifampicin; PZA: pyrazinamide; EMB: ethambutol; SM: streptomycin.
The most frequent ADR was hepatitis. Five of these patients were alcoholic, two had a previous history of chronic liver disease and one was HIV-positive. Asymptomatic hepatotoxicity occurred in 35 cases and the remaining 18 were symptomatic. The most common symptoms were nausea, vomiting and abdominal pain. One female patient with no history of comorbid conditions had a poor outcome and needed liver transplantation while all others had complete liver transaminases normalization.
Rash occurred in five cases, with itchiness in three of them, and was the second most frequent adverse reaction.
One patient with chronic inflammatory arthritis and LTBI suffered gastrointestinal toxicity associated to RIF. One other patient with LTBI and no significant comorbid conditions had an episode of angioedema 25 days after initiating INH plus RIF anti-TB regimen which was subsequently interrupted. EMB was responsible for one single case of ocular toxicity in a patient with genitourinary TB whose visual acuity was never restored.
Side effects emerged mainly after 2 months of anti-TB drug regimen and more than half of these patients had their treatment stopped before completing the total number of doses and had, on average, a total time of treatment 0.7 months less than the predicted one. The remaining had an increase in the overall time of treatment due to one or more interruptions. Only seven cases of side effects during this period had LTBI and the majority had pulmonary TB (n=26).
A small proportion of ADR developed during the initial phase of treatment, that is, before completing 2 months of treatment. Eight of those had a decrease and 11 had an increase in the total number of months of treatment. Nine had LTBI.
It was not possible to identify which specific drug was responsible for the ADR in 82% of the cases since the drug rechallenge occurred with only minor residual effects, allowing treatment to continue, or it was definitively suspended with no further rechallenge. In these situations, all drugs that could have caused that effect were considered responsible.
The combination of INH and RIF was largely responsible for the incidence of side effects, specifically for 41 hepatotoxicity events and 3 cutaneous reactions. Those two drugs plus PZA accounted for six more cases of drug-induced hepatitis.
PZA, INH, RIF, and EMB were responsible for 5, 3, 2 and 1 major side effect, respectively. SM was not associated to any major ADR.
No correlation was found between age or sex and the overall incidence of ADR. However, when considering each category of ADR, younger age was meaningfully related to an increased risk of hepatotoxicity (P=0.035), which was 0.96 times smaller for each year that the age increased. In this group of patients, mean age was 49.0±19.4 years while those that had no drug-related hepatitis had a mean age of 64.6±16.4 years. On the other hand, an important association was found between male gender and toxicity to both INH and PZA but this did not achieve statistical significance (P=0.055).
Diabetes mellitus and major ADR were significantly associated (P=0.042) but the same could not be stated for other comorbidities or multidrug resistance risk factors. One diabetic patient had his treatment with oral anti-diabetic drugs changed to insulin therapy after being diagnosed with TB for an optimal glycaemic control, while the remaining were already being treated with insulin.
DiscussionADR to first-line antituberculosis medication are frequent and have important implications that may affect the effectiveness of treatment, the course of TB disease and chemoprophylaxis in latent infection.
In our study, out of 764 patients treated with first-line antituberculosis drugs for active and latent TB, 55 (7.2%) had at least one major side effect for a total of 61 ADR, which was not as high as in other studies.11,12 The overall incidence of severe ADR was highest in combined treatment with INH and RIF, in patients aged 35 years or older and in the male gender, but none of these variables were statistically significant.
It was only possible to attribute responsibility for the ADR to one particular drug in 18.0% of the cases. The main reason was that when sequential drug rechallenge was implemented, side effects did not recur and the most likely associated drugs were considered probably responsible.
There was a high incidence of hepatotoxicity (6.9%) compared to previous studies performed in both active and latent TB patients.6,12–14 Hepatotoxicity was most likely to be associated to chemotherapy combination of INH and RIF, which has previously been stated to increase the risk of liver injury among patients under antituberculosis treatment compared to regimens containing only one of those drugs.15 A metabolic idiosyncratic mechanism by which additional toxic INH metabolites are produced seems to be the underlying mechanism promoted by RIF to boost hepatotoxicity of other antituberculosis medications.16,17
The overall rate of INH-related hepatitis was 0.3% while that associated to LTBI was 0.1%, which is similar to the rates published in recent literature.18,19
PZA is recognized as the most hepatotoxic among first-line antituberculosis drugs acting on both dose dependent and idiosyncratic mechanisms.6,11,12,20–22 Our findings suggested a slight increase in the incidence of hepatotoxicity (0.8%) when used in combination with INH and RIF with a minor rate of hepatitis attributed to PZA alone (0.5%). Other studies have also shown an increased risk of hepatotoxicity with PZA especially when added to other antituberculosis drugs such as INH and rifampin.23 PZA was also responsible for the one single case of severe hepatotoxicity that progressed to liver transplantation.
Hepatotoxicity causes significant morbidity and mortality, which justifies the numerous studies to define its predisposing factors but results are still controversial. Our series found no correlation between reported risk factors such as preexisting liver disease, high alcohol intake or other comorbidities, and hepatotoxicity. However, this may reflect the overall lack of comorbid conditions in the studied population, which usually has a higher prevalence in patients with hospital follow-up.
Cutaneous rash was the second most frequent ADR with an overall incidence of 8.2%. In fact, cutaneous adverse reactions are commonly observed in patients with antituberculosis drugs and are an important cause of treatment discontinuation.24 Therapeutic regimens with both INH and RIF were mainly responsible for this ADR although most studies suggest that PZA is the most common offending agent.11
Other ADR such as angioedema, gastrointestinal upset and ocular toxicity were rare and each occurred in only one patient, accounting for an overall incidence of 1.6%.
Diabetes was significantly correlated to major ADR. Several studies have suggested that diabetes increases the risk of active TB, especially in the elderly, and leads to worst treatment outcomes.25–29 Also, some small series have shown that metabolic disorders such as diabetes may act as independent risk factors for ADR.30,31 Oral anti-diabetic and antituberculosis drugs may interact and lead to a suboptimal glycaemic control on the one hand, and lower the efficacy of TB treatment on the other hand.32 However, in our study, all diabetic patients were being treated with insulin regimens or started insulin treatment after being diagnosed with TB. Given the increasing worldwide incidence of diabetes our findings support the need for additional clinical surveillance of diabetic patients under antituberculosis treatment.
ADR had a significant repercussion on the overall treatment time. The most frequent change was the extension of the predicted length of treatment that happened with 43.6% of the patients. However, 34.5% of the patients were unable to complete the full treatment due to ADR. Both situations posed a threat for the success of TB treatment and represented a risk of TB treatment failure.
The most striking result was that hepatotoxicity was considerably more frequent in younger patients with an increased risk of 0.96 per year. This diverges from what has been published by preceding reports that suggest that increasing age is a risk factor for hepatotoxicity.11,13,18,19,21,22,33,34 However, many of these studies failed to prove statistical significance and divergent studies show no relationship between the risk of hepatotoxicity and age.35–37 Additionally, similar conclusions to ours were reached by a Turkish group in a study that involved a population of 1149 patients with active TB.12 These results may have been limited by the small amount of patients with hepatotoxicity and aged more than or equal to 60 years (n=15). One possible explanation for our findings is the possible association between younger age and high alcohol intake, frequently neglected due to often-overlooked consumption habits among this population.
Our study, as all retrospective studies, has intrinsic limitations. The small number of patients with major ADR and the comparison with a minor matched control-group provided limited power to detect significant associations with most of the comorbidities and MDR risk factors that were found in few patients. Furthermore, patients treated on an outpatient basis in itself constitutes a selected population with less comorbid conditions and milder forms of the disease.
However, our analysis stands out because it includes a wide spectrum of patients submitted to anti-TB treatment, not only with pulmonary or extrapulmonary TB but also LTBI, all treated at single centre. The study design is superimposable to the one related in other series and therefore comparisons can be done.
ConclusionsThe development of novel antituberculosis drugs in the middle of the 20th century increased the rate of successfully treated tuberculosis cases. This has, on the other hand, increased the number of drug-related side effects, some of which may significantly interfere with the therapeutic regimen and the whole treatment time.
This study found a high frequency of ADR which had a strong impact on subsequent therapeutic orientation. Therefore, preliminary clinical assessment and subsequent close monitoring, including laboratory routine analysis, are extremely important for, on the one hand, identification of risk factors and, on the other hand, for early recognition of ADR, assessing the side-effects severity and making prompt treatment decisions.
Of particular interest is the relationship between ADR and diabetes mellitus and the increased frequency of hepatotoxicity at a younger age. Thus, an extra careful follow-up of these patients is fundamental to avoid subsequent treatment failures.
New antituberculosis drugs are needed, not only to simplify and improve treatment, but also to reduce the incidence of important side effects.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.