The diagnosis of a pancreatopleural fistula (PPF) is frequently delayed, because this is a rare condition and is often asymptomatic.1 The presence of a large volume pleural effusion with high protein and pancreatic enzymes content, that recurs after chest tube drainage, may be its only manifestation.2
A 43-year-old male, HIV positive on combined antiretroviral therapy, presented to our emergency department (ED) with sudden effort dyspnea and no other associated symptoms. Physical examination revealed diminished respiratory sounds on the left lung. Chest X-rays showed a left, large volume pleural effusion.
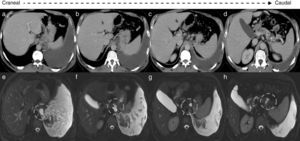
Thoracoabdominal CT scan demonstrated a large volume left pleural effusion and signs of chronic pancreatitis (multiple diffuse calcifications, Wirsung dilatation) and a small pancreatic fluid collection adjacent to the Wirsung in the body–tail transition (Fig. 1d). Although very discrete, one could hardly visualize a thin fistulous tract communicating this collection with the left pleural cavity (Fig. 1a–d).
(a)–(d) Axial thoracoabdominal CT scan: large volume left pleural effusion; signs of chronic pancreatitis-diffuse pancreatic calcifications, (d) pancreatopleural fistulous tract – dashed circles. (e)–(h) MRCP axial planes: allow for a better depiction of the fistulous tract communicating with the pleural space through the aortic hiatus – dashed circles.
Drainage of the pleural fluid revealed a hematic exudate, with an amylase concentration of 5645U/L.
Magnetic resonance cholangiopancreatography (MRCP), performed 5 days after admission, confirmed the diagnosis, allowing a better depiction of the fistulous tract, which was originated in the body–tail transition, in a small pancreatic collection in continuity with the main pancreatic duct. The fistulous tract extended toward the chest, communicating with the left pleural space through the aortic hiatus.
PPFs are estimated to occur in around 0.4% of all the pancreatitis and in 4.5% of patients with a pancreatic pseudocyst.3
Some authors suggest that the number of diagnosed or reported cases is underestimated and will tend to increase with progressive improvement of imaging techniques.4
Patients with PPFs are predominantly (83%) men, on their fourth decade of life, with chronic pancreatitis usually associated with long-term alcohol consumption.5
The most common symptoms are related to the pleural effusion and include dyspnea (65%), cough (29%) and chest pain (27%).5 Abdominal pain has been reported in 23% of the cases.5
According to the literature, CT scan is the preferred imaging technique, allowing for the identification of the fistulous tract in 33–47% of cases.4–6
MRCP is also a method of choice for the diagnosis of PPF, with a sensitivity of 80% and a good alternative to CT. It has the advantage of being noninvasive and able to identify PPF even in the context of severe pancreatic duct stricture.4,5,7
It allows the recognition of ductal anatomy and pathologic changes of the surrounding structures, yielding important information for a better understanding of local anatomy and treatment planning.4,7
In the reported case, MRCP led, in fact, to the confirmation of the fistulous tract suspected in CT and the identification of its origin on a ductal stenotic component near the pancreatic body–tail transition.
In this case, treatment consisted of several thoracentesis for pleural fluid evacuation and subsequent chest drain placement due to pleural effusion persistence. Somatostatin analogs and parenteral nutrition were also part of the treatment strategy.
During the first week of treatment there was significant worsening of dyspnea, and an increase of the pleural effusion on X-ray. A new CT scan showed marked enlargement of the pleural effusion, with collapse of both left lung lobes and right deviation of the mediastinum.
An endoscopic retrograde colangiopancreatography (ERCP) was performed, with Wirsung duct sphincterotomy for decompression.
Medical conservative treatment of PPF is usually maintained for 2–3 weeks.1 In this case, given the immunodepressed state of the patient, surgery was postponed.
After 5 weeks of conservative treatment, there was no significant regression of the pleural effusion and a purulent fluid through the thoracic drain was observed. Surgical treatment was then employed, with a body–tail pancreatectomy and Y-Roux pancreatojejunostomy. After surgery the pleural effusion improved significantly and 10 days later the patient was discharged with no dyspnea, pain or other symptoms.
In conclusion, pancreatopleural fistulas must be taken into account in cases of large volume pleural effusion in patients with a history of pancreatitis or pancreatic surgery. Advanced imaging techniques, like CT and especially MRCP, allows for the direct visualization of fistulous tracts and for the establishment of the diagnosis, yielding important information for a better understanding of local anatomy and treatment planning.
Conflicts of interestThe authors have no conflicts of interest to declare.
A special word of acknowledgment to Dr. Ana Sofia Figueiredo for her precious help in the elaboration of this paper.