While the need to implement evidence-based training following COVID-19 is imperative, no consensus exists as to how such programmes should be designed.1-3 Between 20 July 2020 and 30 April 2021, we assessed tolerability and safety of high-intensity constant-load exercise (CLE) and high-intensity intermittent exercise (IE) in 14 patients presenting pneumonia and acute respiratory failure (ARF) (mean age: 63±13 years) with ongoing symptomatic COVID-19 from 4 to 12 weeks following the infection. Anthropometric data, body mass index (BMI), and the number of comorbidities were recorded. Patients undertook spirometry (FEV1, FVC, FEV1/FVC, transfer factor for Carbon Monoxide (DLCO), blood gases in room air (PaO2, PaCO2, pH), functional status (the Short Physical Performance Battery-SPPB test and the 6-minute walking distance [6MWD: in meters and as a percentage of predicted]), an incremental cardiopulmonary exercise test (CPET) [assessing oxygen uptake (VO2), carbon dioxide output (VCO2), oxygen uptake at the anaerobic threshold (AT), respiratory exchange ratio (RER), minute ventilation (VE), tidal volume (VT) and respiratory rate] using a portable metabolimeter; transcutaneous carbon dioxide tension (TcCO2) was also recorded continuously. In this crossover study (Ethics Committee approval on 30 June 2020, Procotol No. 2449CE), training exercise intensity was balanced to provide the same average work rate for IE and CLE modalities. CLE was set at 70% of peak work rate (WRpeak) and IE consisted of one minute of exercise at 100% WRpeak, alternated with one minute at 40% WRpeak, to the limit of tolerance (Tlim). Dyspnea and leg muscle discomfort (1-10 Borg scale), heart rate and safety were assessed. Of the 220 consecutively admitted patients at the Respiratory Rehabilitative Unit - ICS Maugeri of Lumezzane (BS) - as inpatient and outpatient between 20 July 2020 and 30 April 2021, 14 patients were eligible for the study. We excluded from this study 63 patients presenting symptoms for less than four weeks following infection, 35 patients with more than 12 weeks following infection, 44 clinically unstable patients, 20 patients with severe orthopedic diseases, 15 patients with cognitive impairment, 29 patients with previous severe heart disease (congestive heart disease, severe aortic stenosis, atrial fibrillation). We did not successively exclude patients for technical reasons or missing data.
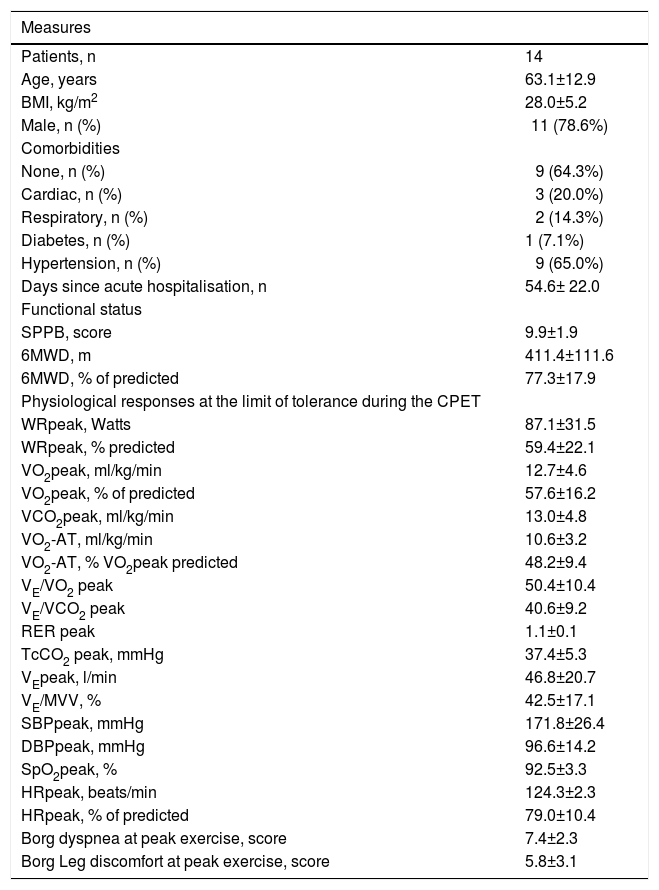
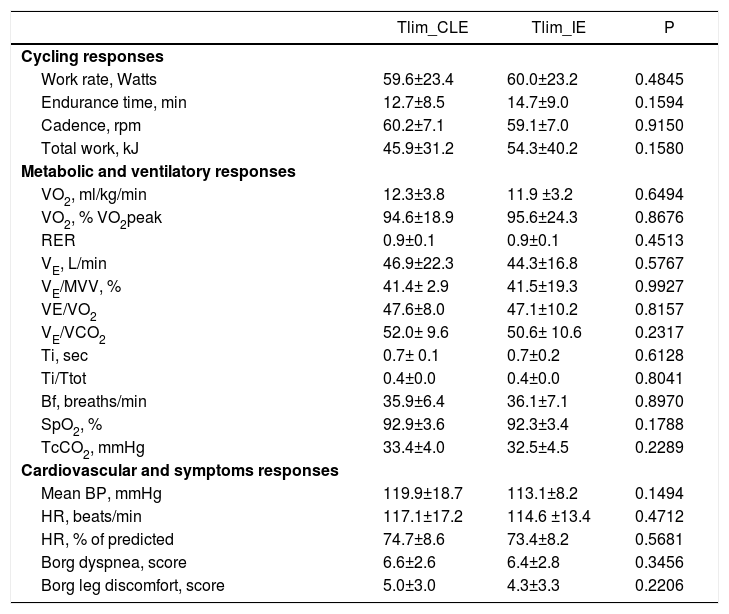
Table 1 shows the study population and cardiorespiratory function at peak exercise; two patients presented mitral valve insufficiency and one chronic atrial fibrillation, while two patients suffered from mild COPD. At study entry, patients showed breathlessness (71.4%), fatigue (64.3%), cough (14.4%), palpitations (21.4%) and pain (35.7%), respectively. Patients presented the following lung function data: FEV1 % predicted (prd): 83.2±15.7, FVC, % prd: 79.1±15.8., FEV1/FVC: 84.1±8.5, DLCO % prd: 56.7±26.6, PaO2: 73.7±11.8 mmHg, PaCO2: 36.9±3.01 mmHg. We reported no adverse events for either of the two modalities. We detected no ECG abnormalities during or after IE or CLE. At peak exercise, WRpeak and V̇O2peak were reduced below normal predicted levels. Premature metabolic acidosis was evident by the low fraction of predicted normal VO2 when the anaerobic threshold (AT) was detected (AT at 48±9% VO2 prd). Overall, respiratory reserve was not exhausted in patients with COVID-19. Ventilatory equivalents for VO2 (VE/VO2) and VCO2 (VE/VCO2), and transcutaneous carbon dioxide tension (TcCO2) were compatible with exercise hyperventilation (Table 1). A recent study in survivors from COVID-19 pneumonia has suggested that exercise hyperventilation after COVID-19 is frequent and principally due to enhanced chemoreflex sensitivity rather than increased VD/VT.4 We observed a mild reduction in arterial oxygen saturation (SpO2). Arterial blood pressure was normal, whereas the mean heart rate reached approximately 80% of predicted normal value. Sensations of breathlessness and leg discomfort were indicative of severe symptoms. The predominant symptom for stopping exercise was breathlessness (6/14), leg discomfort (2/14) or both dyspnoea and leg discomfort (6/14). Exercise endurance time was not different between IE compared to CLE (p = 0.1594, Table 2). The average cycling work rate did not differ between IE and CLE. The same was also the case for VO2 and for both ventilatory equivalents (Table 2). At the limit of cycling tolerance, none of the ventilatory or cardiovascular responses differed between IE and CLE (Table 2) and there was no difference in the intensity of breathlessness or leg discomfort between the two modalities. The ventilatory reserve, reflected by the ratio of VE/maximal voluntary ventilation (VE/MVV), did not differ between IE and CLE. During CLE and IE 36% and 21% of patients, respectively, ended the test with a HR greater than 80% of maximal predicted. Forty-three percent of patients ended CLE and 50% ended IE with a decrease in SpO2 greater than 4%, compatible with exercise-induced arterial oxygen desaturation. The fraction of patients who reasoned dyspnoea as the limiting factor was identical between IE and CLE corresponding to 57%. The fraction of patients who stopped exercise because of leg discomfort was relatively low for IE (n=2, 14%) and for CLE (n=3; 21%). Both dyspnoea and leg discomfort as the limiting factors were reported by n=4 for IE (29%) and n=3 for CLE (22%). At exercise iso-time and the limit of tolerance during IE and CLE protocols, VE, SPO2 and VO2 did not differ (Table 2). Moreover, symptoms for breathlessness, leg discomfort, heart rate or blood pressure measurements were not different during IE and CLE protocols.
Demographic, anthropometric and clinical characteristics.
Legend: Results are expressed as mean± Standard Deviation; BMI, body mass index; SPPB, Short Physical Performance Battery; 6MWD, six minute walking distance; CPET, cardiopulmonary exercise test; WRpeak, maximum load in watts at peak exercise; VO2, oxygen uptake; VE, ventilation; SpO2, peripheral oxygen saturation; TcCO2, transcutaneous carbon dioxide tension; VO2-AT, oxygen uptake at the anaerobic threshold; RER, respiratory exchange ratio; VE/VO2; ventilatory equivalent for VO2, VE/VCO2, ventilatory equivalent for VCO2; SBP: systolic blood pressure; DBP: diastolic blood pressure; HR, heart rate.
Responses at the limit of tolerance (Tlim) to constant-load exercise (CLE) and interval exercise (IE) protocols.
Legend: Results are expressed as mean± Standard Deviation; VO2, oxygen uptake; VE, minute ventilation; arterial oxygen saturation; TcCO2, transcutaneous carbon dioxide; CPET, cardiopulmonary exercise test; RER, respiratory exchange ratio; VE/VO2, ventilatory equivalent for VO2; VE/VCO2, ventilatory equivalent for VCO2; BP, blood pressure; HR, heart rate.
The lack of adverse events occurring during exercise modalities was in line with previous studies on COVID-19 survivors.5 Moreover, several studies on other ‘high risk’ patients’ groups (e.g., such as ischemic heart disease and heart failure) showed that high-intensity exercise is considered safe.6,7 Recent studies in COVID-19 survivors4,8 have attributed early metabolic acidosis to myopathic changes occurring for medications administered during the hospital stay (e.g., steroids) as well as because of the potential direct or indirect myopatic damage from COVID-19 rather than muscle disuse.7 Hence, several opinion papers and guidelines favour low-intensity exercise with gradual increases in intensity, mostly due to safety concerns.2,3 Early experiences of rehabilitation in post-COVID-195 individuals show that low-to-moderate intensity of exercise in this population is safe and effective in improving exercise tolerance and peripheral muscle strength. Accordingly, our study was designed to investigate the safety and tolerability of high-intensity (continuous or interval) exercise in this population.
Individuals with ongoing symptomatic COVID-19 could successfully and safely undertake high-intensity exercise performed continuously or intermittently. These findings are relevant both for a better understanding of consequences of COVID-19 on exercise tolerance. They also provide a clearer suggestion to survivors on how they should undertake regular exercise when expecting to resume their previous lifestyle.
AcknowledgmentsThe authors thank Laura Comini and Adriana Olivares for technical assistance. This work was supported by the “Ricerca Corrente” Funding scheme of the Ministry of Health, Italy.
Author contributionsMP (Guarantor) had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. MV, IV, MP and MVe contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript. BS, LB contributed to data collection and interpretation. All Authors reviewed the manuscript.