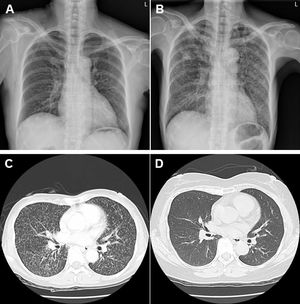
A 51-year-old woman had a diagnosis of seropositive rheumatoid arthritis (RA) with polyarthritis involving bilateral elbow, small joints of hand and knee areas on March 12, 2004. Owing to refractory therapeutic responses with a DAS28 score of 6.16 under methotrexate 15mg/week, hydroxychloroquine 400mg/day and prednisolone 10mg/day, she started to receive regular biweekly 40mg subcutaneous injection of adalimumab, a tumor necrosis factor (TNF) monoclonal antibody (mAb) since June 17, 2011. She had no history of lung diseases with a clear chest X-ray (Fig. 1A). There was a negative QuantiFERON test before initiating anti-TNFtherapy. She had reduced disease activity after treatment without further prescription of hydroxychloroquine and prednisolone. Owing to productive cough with intermittent fever and weight loss for 3 weeks, the use of TNF mAb was discontinued on February 20, 2018, 80 months after initiating such a treatment. However, she did not receive follow-up QuantiFERON test during her long-term anti-TNF therapy. Chest images including X-ray (Fig. 1B) and computed tomography (Fig. 1C) showed bilateral diffuse miliary lesions characterized by innumerable micronodular opacities. She had no exposure to fine mineral or chemical dust, and her malignancy survey and human immunodeficiency virus examination were negative results. Sputum cultures were positive for Mycobacterium tuberculosis. There was no recent household tuberculosis (TB) contact history. She received anti-TB medications with daily dosages of rifampicin 480mg, isoniazid 320mg, pyrazinamide 1000mg and ethambutol 800mg for 9 months from April 12, 2018 (drug-susceptibility testing in TB with resistance to streptomycin and sensitive to others). After antibiotic therapy, there were no more respiratory or constitutional symptoms, negative sputum culture results and resolved pulmonary miliary nodules (Fig. 1 D). For her arthritis flare-up after discontinuing TNF mAb injection, rituximab, a B-cell depleting mAb, under a regimen of 1g×two fortnightly infusions every 6 months was initiated on February 7, 2020 with improved disease activity (DAS 28 score 5.34 to 2.70) after 4 infusions at the end of 2020.
Clinical images of miliary TB in a RA patient receiving TNF mAb therapy. (A). A clear chest X-ray without any lung lesions before starting TNF mAb therapy. (B). Bilateral diffuse military lesions on chest X-ray after TNF mAb therapy. (C). Bilateral innumerable micronodular opacities on chest computed tomography after TNF mAb therapy. (D). Resolved bilateral military nodules on chest computed tomography after anti-TB treatment.
Miliary TB, a rare fatal presentation of mycobacterial infection, can occur in the presence of impaired host immunity under the prescription of immunosuppressive agents.1,2 TNF blocker administration has been recognized as a risk for TB reactivation in various autoimmune-mediated inflammatory disorders,3 especially when anti-TNF treatment is combined with the use of immunosuppressive agents like methotrexate.4 Greater incidences are found during the first year of treatment than afterwards, while there are higher occurrences with mAb than with recombinant soluble receptor fusion protein therapy. Despite an additional risk of having been born in an area of endemic TB,5 the reported case raises a potential infection hazard under the long-term TNF mAb treatment.
In conclusion, we reported miliary TB in a RA patient receiving long-period TNF blocker therapy. In addition to the initial QuantiFERON survey, periodic latent TB testing should be carried out in patients receiving the long-term immunosuppressive agents like TNF monoclonal antibodies.
Author contributionsStudy conception and design: Chrong-Reen Wang.
Data analysis and acquisition: Chrong-Reen Wang, Wei-Chieh Lin, Yi-Shan Tsai.
Drafting of the manuscript: Chrong-Reen Wang.
All authors had access to the data and played a role in writing this manuscript.
FundingThis study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflicts of interest to declare.
This study was approved by the Institutional Review Board (B-ER-105-108) with informed consent from the patient.